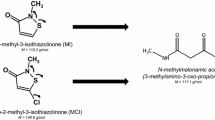
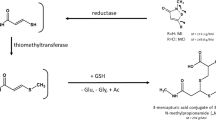
Summary
During 5 days following a single oral dose of 3H-11-bromovincamine (40 mg) to two human subjects, means of 55% and 27% of the3H dose were excreted in the urine and faeces respectively, mainly within 24 and 48 h.
Mean plasma concentrations of3H reached a peak (1900 ng equiv./ml) at 1 h after dosing and declined biphasically with half-lives of 5 h and 11 h which were similar to half-lives for urinary excretion of3H. Parent drug and 11 -bromovincaminic acid were the major dose-related components in plasma at 1.5 and 3 h. Mean plasma concentrations of 11-bromovincamine reached a peak (620 ng/ml) at 0.75 h and declined biphasically with half-lives of about 1 h and 5 h.
The major urinary metabolite was 11-bromovincaminic acid (31% dose). Also present in urine were 11-bromovincamine (3%), 11-bromoapovincamine (1%) and 2 unknown metabolites (9% and 6%). Similar metabolites were detected in faecal extracts.
If inadequately stored in biological samples, 11-bromovincamine could be hydrolysed to 11-bromovincaminic acid and be epimerised to 11-bromo-epivincamine.
Similar content being viewed by others
References
Szporny L. (1977): Pharmacologie de la vincamine et de ses dérivés. Actualités pharmacologiques,39, 87–117.
Mayo B.C., Biggs S.R., Hawkins D.R., Chasseaud L.F., Darragh A., Baidock G.A. and Whitby B.R. (1982): The metabolic fate of 11-bromo-[15-3H]vincamine in rats, dogs and humans. J. Pharm. Dyn.,5, 951–964.
Mayo B.C., Biggs S.R., Chasseaud L.F., Hawkins D.R., Darragh A. and O’Kelly D.A. (1980): The metabolic fate of sormodren (bornaprine hydrochloride) in animals and humans. Xenobiotica,10, 873–888.
Cameron B.D., Chasseaud L.F., Hawkins D.R. and McCormick D.J. (1976): The absorption of [G-3H]acetylglycyrrhetate after oral administration to rats. Arzneim-Forsch.,26, 1680–1683.
Mayo B.C., Brodie R.R., Chasseaud L.F. and Hawkins D.R. (1978): Biotransformation of methyl 5-cyclopropylcarbonyl-2-benzimidazole carbamate (ciclobendazole) in rats and dogs. Drug Metab. Disposition,6, 518–527.
Brodie R.R. and Chasseaud L.F. (1982): Determination of 11-bromovincamine in human plasma by high-performance liquid chromatography. J. Chromatogr.,228, 413–417.
Vereczkey L., Czira G., Tamas J., Szentirmay Zs., Botar Z. and Szporny L. (1979): Pharmacokinetics of vinpocetine in humans. Arzneim-Forsch.,29, 957–960.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mayo, B.C., Biggs, S.R., Hawkins, D.R. et al. The metabolic fate of 11-bromo[15-3H]vincamine in man. European Journal of Drug Metabolism and Pharmacokinetics 10, 189–196 (1985). https://doi.org/10.1007/BF03189741
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03189741