Abstract
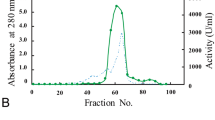
The aim of the present study was to purify and characterize angiotensin-converting enzyme (ACE) present in frog ovary (Rana esculenta). Detergent and trypsin-extracted enzymes were purified using a one-step process, consisting of affinity chromatography on lisinopril coupled to Sepharose 6B. The molecular mass was 150 kDa for both detergent-extracted and trypsin-extracted enzyme. The specific activity of detergent-extracted and trypsin-extracted ACE was 294 U mg−1 and 326 U mg−1 respectively. The optimum pH range was from 7–8.5 at 37°C and the optimum temperature was 50 °C. Optimum chloride concentration was about 200 mM for synthetic substrate FAPGG (N-[3-(2-furyl)acryloyl] L-phenylalanyl glycyl glycine) and angiotensin I, and 10 mM for bradykinin. The Km and Kcat values for FAPGG were 0.608±0.07 mM and 249 sec−1 respectively and I50 values for captopril and lisinopril, two specific ACE inhibitors, were 68±12.55 nM and 6.763±0.66 nM respectively. Frog ovary tissue from prereproductive period was incubated in vitro in the presence of frog ovary ACE (2.5 mU/ml), captopril (0.1 mM), and lisinopril (0.1 mM). Production of 17β-estradiol, progesterone, and prostaglandins E2 and F2α was determined. The data showed a modulation of 17β-estradiol, progesterone and prostaglandin E2 production by ovary ACE.
Resumen
La finalidad de este estudio es la purificación y caracterización de la enzima convertidora de angiotensina (ACE) contenida en el ovario de rana (Rana esculenta). La enzima, extraída tanto con detergente como con tripsina, se purificó mediante un proceso único, consistente en una cromatografía de afinidad con lisinopril ligado a Sepharose 6B. El peso molecular de la enzima resultó ser de 150 kDa, tanto por la extracción con detergente como con tripsina. La actividad específica de la ACE extraída con detergente y tripsina fue de 294 U mg−1 y 326 U mg−1, respectivamente. El intervalo de pH óptimo fue de 7–8,5 a 37°C y la temperatura óptima, de 50 °C. La concentración molar óptima de cloruro fue sobre 200 mM para el sustrato sintético FAPGG (N-[3-(2-furyl) acryloyl] L-phenylalanyl glycyl glycine) y angiotensina I, y 10 mM para la bradiquinina. Los valores de Km y Kcat para FAPGG fueron 0,608±0,07 mM y 249 sec−1, respectivamente, y los valores de I50 para captopril y lisinopril, dos inhibidores específicos de ACE, fueron 68±12,55 nM y 6,763±0,66 nM, respectivamente. Tejido ovárico de rana en períodos prerre-productivos se incubó in vitro en presencia de ACE de ovario de rana (2,5 mU/ml), captopril (0,1 mM), y lisinopril (0,1 mM), determinán-dose la producción de 17β-estradiol, progesterona y de prostaglandinas E2 y F2α. Los resultados muestran modulación de la producción de 17β-estradiol, progesterona y prostaglandinas E2 por ACE ovárica.
Similar content being viewed by others
References
Bradford, M. (1976): Anal. Biochem., 72, 248–254.
Bramucci, M., Miano, A., Gobbetti, A., Zerani, M., Quassinti, L., Maccari, E., Murri, O. and Amici, D. (1997): Am. J. Physiol., 273, R2089-R2096.
Bull, H. G., Thornberry, N. A., Cordes, M. H. J., Patchet A. A. and Cordes, E. H. (1985): J. Biol. Chem., 260, 2952–2962.
Buttery, J. E. and Stuart, S. (1993): Clin. Chem., 39, 312–316.
D’Istria, M., Delrio, G., Botte, V. and Chieffi, G. (1974): Steroids Lipids Res., 5, 42–48.
Gobbetti, A. and Zerani, M. (1995): J. Endocrinol., 145, 235–241.
Hasegawa, Y., Watanabe, T. X., Sokabe, H. and Nakajima, T. (1983): Gen. Comp. Endocrinol., 50, 75–80.
Hopper, N. M. and Turner, A. J. (1987): Biochem. J., 241, 625–633.
Laemmli, U. K. (1970): Nature, 227, 680–685.
Lipke, D. W. and Olson, K. R. (1988): Physiol. Zool., 61, 420–428.
Miano, A., Bramucci, M., Murri, O., Quassinti, L., Maccari, E., Zerani, M., Gobbetti, A. and Amici, D. (1997): Acta Physiol. Scand., 160, 277–282.
Ondetti, M. A., Rubin, B. and Cushman, D. W. (1977): Science, 196, 441–444.
Peach, M. J. (1977): Physiol. Rev., 57, 313–370.
Quassinti, L., Miano, A., Bramucci, M., Maccari, E. and Amici, D. (1998): Biochem. Mol. Biol. Int., 44, 887–895.
Rieger, K. J., Saez-Servent, N., Papet, M. P., Wdzieczak-Bakala, J., Morgat, J. L., Thierri, L., Voelter, W. and Lenfant, M. (1993): Biochem. J., 296, 373–378.
Sabeur, K., Vo, A. T. and Ball, B. A. (2001): J. Reprod. Fertil., 122, 139–146.
Smiley, J. W and Doig, M. T. (1994): Comp. Biochem. Physiol., 108, 491–496.
Sokal, R. R. and Rohlf, F. J. (1981): Biometry, 2nd ed. Freeman, San Francisco, CA.
Wilson, J. X. (1984): Endocr. Rev., 5, 45–61.
Wray, W., Boulikas, T., Wray, V. P. and Hancock, R. (1981): Anal. Biochem., 118, 197–203.
Yamaguchi, T., Kurihara, S., Ikekita, M., Kiziki, K. and Moriya, H. (1986): J. Pharmacobio-Dyn., 9, 585–592.
Yang, H. Y., Erdos, E. G. and Levin, Y. (1970): Biochim. Biophys. Acta, 214, 374–376.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miano, A., Quassinti, L., Maccari, E. et al. Purified angiotensin converting enzyme from Rana esculenta ovary influences ovarian steroidogenesis in vitro . J. Physiol. Biochem. 59, 269–276 (2003). https://doi.org/10.1007/BF03179884
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03179884