Abstract
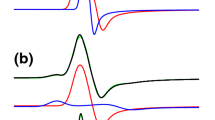
Electron paramagnetic resonance (EPR) and proton ENDOR (electron-nuclear double resonance) spectroscopies were used to analyze the structural and electronic parameters of the oxidized [Fe4S4] cubane clusters in high-potential iron sulfur proteins (HiPIPs) fromEctothiorhodospira halophila (HiPIP I) andRhodocyclus tenuis. TheE. halophila HiPIP I EPR spectra at X- and Q-band revealed a dominant species (simulated withg max=2.1425,g int=2.0315,g min=2.0296) and a minor species (ca. 5–10% contribution) which was not analyzed further. ForR. tenuis HiPIP the EPR spectrum contained a single species only (g max=2.1140,g int=2.0392,g min=2.0215), i.e., withg max significantly smaller than that of theE. halophila protein. Orientation-selected proton ENDOR spectra of HiPIP I ofE. halophila were reconstructed by simulation with slight modifications of the crystal structure data. ENDOR from two mutants, F36S and F36G, ofE. halophila HiPIP I gave evidence for a common assignment of a HB2 proton of a phenylalanine residue (36 and 44, respectively, in isoenzymes I and II as reported earlier) interacting with the mixed-valence pair iron ions Fe2 and Fe3. ForR. tenuis HiPIP, the ENDOR spectra were assigned to arise from Fe3 and Fe4 as mixedvalence pair under the assumption of an unchanged intrinsicg-tensor symmetry. The resulting site specificity of the cubane oxidation was discussed in relation to structural requirements and redox potentials of the two HiPIPs.
Similar content being viewed by others
References
Bentrop D., Capozzi F., Luchinat C. in: Handbook on Metalloproteins (Bertini I., Sigel A., Sigel H., eds.), p. 357. New York: Marcel Dekker 2001.
Noodleman L.: Inorg. Chem.27, 3677–3679 (1988)
Mouesca J.M., Rius G., Lamotte B.: J. Am. Chem. Soc.115, 4717–4731 (1993)
Mouesca J.M., Noodleman L., Case D., Lamotte B.: Inorg. Chem.34, 4347–4359 (1995)
Belinskii M., Bertini I., Galas O., Luchinat C.: Z. Naturforsch. A50, 75–80 (1995)
Dugad B., La Mar G.N., Banci L., Bertini I.: Biochemistry29, 2263–2271 (1990)
Fritz J., Anderson R., Fee J., Palmer G., Sands R.H., Tsibris J.C.M., Gunsalus I.C., Orme-Johnson W.H., Beinert H.: Biochim. Biophys. Acta253, 110–133 (1971)
Canne C., Ebelshäuser M., Gay E., Shergill J.K., Cammack R., Kappl R., Hüttermann J.: J. Biol. Inorg. Chem.5, 514–526 (2000)
Kappl R., Ebelshäuser M., Hannemann F., Bernhardt R., Hüttermann J.: Appl. Magn. Reson.30, 427–459 (2006)
Gurbiel R.J., Batie C.J., Sivaraja M., True A.E., Fee J.A., Hoffman B.M., Ballou D.P.: Biochemistry28, 4861–4871 (1989)
Banci L., Bertini I., Capozzi F., Carloni P., Ciurli S., Luchinat C., Piccioli M.: J. Am. Chem. Soc.115, 3431–3440 (1993)
Kappl R., Ciurli S., Luchinat C., Hüttermann J.: J. Am. Chem. Soc.121, 1925–1935 (1999)
Bertini I., Capozzi F., Eltis L.D., Felli I.C., Luchinat C., Piccioli M.: Inorg. Chem.34, 2516–2523 (1995)
Dilg A.W.E., Mincione G., Achterhold K., Iakovleva O., Mentler M., Luchinat C., Bertini I., Parak F.G.: J. Biol. Inorg. Chem.4, 727–741 (1999)
Gloux J., Gloux P., Lamotte B., Mouesca J.M., Rius G.: J. Am. Chem. Soc.116, 1953–1961 (1994)
Antanaitis B.C., Moss T.: Biochim. Biophys. Acta405, 262–279 (1975)
Babini E., Bertini I., Borsari M., Capozzi F., Dikyi A., Eltis L.D., Luchinat C.: J. Am. Chem. Soc.118, 75–80 (1996)
Dilg A.W.E., Capozzi F., Mentler M., Iakovleva O., Luchinat C., Bertini I. Parak F.G.: J. Biol. Inorg. Chem.6, 232–246 (2001)
Priem A.H., Klaassen A.A.K., Reijerse E.J., Meyer T.E., Luchinat C., Capozzi F., Dunham W.R., Hagen W.R.: J. Biol. Inorg. Chem.10, 417–424 (2005)
Eltis L.D., Iwagami S.G., Smith M.: Protein Eng.7, 1145–1150 (1994)
Caspersen M.B., Bennett K., Christensen H.E.M.: Protein Expr. Purif.19, 259–264 (2000)
Hüttermann J., Däges G.P., Reinhard H., Schmidt G.: Nuclear Magnetic Resonance of Paramagnetic Macromolecules, p. 165. Dordrecht: Kluwer 1995.
Hüttermann J. in: EMR of Paramagnetic Molecules (Berliner L.J., Reuben J., eds.). Biological Magnetic Resonance, vol. 13, p. 219. New York: Plenum 1993.
Hurst G.C., Henderson T.A., Kreilick R.W.: J. Am. Chem. Soc.107, 7294–7299 (1985)
Breiter D.R., Meyer T.E., Rayment I., Holden H.M.: J. Biol. Chem.266, 18660–18667 (1991)
Rayment I., Wesenberg G., Meyer T.E., Cusanovich M.A., Holden H.M.: J. Mol. Biol.228, 672–686 (1992)
Przysiecki C.T., Meyer T.E., Cusanovich M.A.: Biochemistry24, 2452–2594 (1985)
Heering H.A., Bulsink Y.B.M., Hagen W.R., Meyer T.E.: Biochemistry34, 14675–14686 (1995)
Menin L., Gaillard J., Parot P., Schoepp B., Nitschke W., Verméglio A.: Photosynth. Res.55, 343–348 (1998)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kappl, R., Eltis, L.D., Caspersen, M.B. et al. Site-specific oxidation of the [Fe4S4] cubanes in high-potential iron sulfur proteins as probed by EPR and orientation-selective proton ENDOR spectroscopy:Ectothiorhodospira halophila I versusRhodocyclus tenuis . Appl. Magn. Reson. 31, 483–507 (2007). https://doi.org/10.1007/BF03166598
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03166598