Abstract
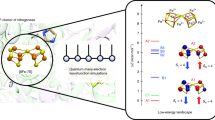
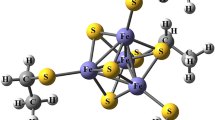
The Thermus thermophilus Rieske protein (TtRP) contains a 2Fe-2S cluster with one iron (Fe-Cys) coordinated by four sulfur atoms (2xS2− and 2xCys) and one iron (Fe-His) by two sulfur and two nitrogen atoms (2xS2−, His134 and His154). Here, the protein is investigated at three pH values (6.0, 8.5 and 10.5) in order to elucidate the protonation states of the His-ligands. Examination of the effect of protonation on the electronic structure of the cluster via Mössbauer spectroscopy gives a deeper understanding of the coupling of electron transfer to the protonation state of the His-ligands. Two components (1 referring to Fe-Cys and 2 to Fe-His) with parameters typical for a diamagnetic [2Fe-2S]2+ cluster are detected. The Mössbauer parameters and the protonation state clearly correlate: while δ remains almost pH-independent with δ 1 (pH6.0) = 0.23 (± 0.01) mms− 1 and δ 1 (pH10.5) = 0.24 (± 0.01) mms− 1 for Fe-Cys, it decreases for Fe-His from δ 2 (pH6.0) = 0.34 (± 0.01) mms− 1 to δ 2 (pH10.5) = 0.28 (± 0.01) mms− 1. ΔE Q changes from ΔE Q1 (pH6.0) = 0.57 (± 0.01) mms− 1 to ΔE Q1 (pH10.5) = 0.45 (± 0.01) mms− 1 and from ΔE Q2 (pH6.0) = 1.05 (± 0.01) mms− 1 to ΔE Q2 (pH10.5) = 0.71 (± 0.01) mms− 1. Density functional theory (DFT)-calculations based on the crystal structure (pdb 1NYK) (Hunsicker-Wang et al. Biochemistry 42, 7303, 2003) have been performed for the Rieske-cluster with different His-ligand protonation states, reproducing the experimentally observed trend.
Similar content being viewed by others
References
Hunsicker-Wang, L.M., Heine, A., Chen, Y., Luna, E.P., Todaro, T., Zhang, Y.M., Williams, P.A., McRee, D.E., Hirst, J., Stout, C.D., Fee, J.A.: Biochemistry 42, 7303 (2003)
Rassow, J., Hauser, K., Netzker, R., Deutzmann, R.: Biochemie. Georg Thieme Verlag KG, Stuttgart (2012)
Link, T.A.: FEBS Lett. 412, 257 (1997)
Hsueh, K.-L., Westler, W.M., Markley, J.L.: J. Am. Chem. Soc. 132, 7908 (2010)
Konkle, M.E., Muellner, S.K., Schwander, A.L., Dicus, M.M., Pokhrel, R., Britt, R.D., Taylor, A.B., Hunsicker-Wang, L.M.: Biochemistry 48, 9848 (2009)
Frisch, M.J., et al.: Gaussian 16, Revision A.03. Gaussian, Inc., Wallingford CT (2016)
Neese, F.: Softw. Focus 2, 73 (2012)
Kuila, D., Fee, J.A.: J. Biol. Chem. 261, 2768 (1986)
Fee, J.A., Findling, K.L., Yoshida, T., Hille, R., Tarr, G.E., Hearshen, D.O., Dunham, W.R., Day, E.P., Kent, T.A., Münck, E.: J Biol. Chem. 259, 124 (1983)
Albers, A., Demeshko, S., Dechert, S., Saouma, C.T., Mayer, J.M., Meyer, F.: J. Am. Chem. Soc. 136, 3946 (2014)
Leggate, E.J., Bill, E., Essigke, T., Ullmann, G.M., Hirst, J.: PNAS 101, 10913 (2004)
Gütlich, P., Bill, E., Trautwein, A.X.: Mössbauer Spectroscopy and Transition Metal Chemistry. Springer, Berlin (2011)
Riedel, E., Janiak, C.: Anorganische Chemie. Walter de Gruyter GmbH & Co. KG, Berlin (2011)
Johnson, C.R., Shepherd, R.E., Marr, B., O’Donnell, S., Dressick, W.: J. Am. Chem. Soc. 102, 6227 (1980)
Acknowledgements
This work is supported by the German Research Foundation (DFG) under SPP 1927 (SCHU 1251/17-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Proceedings of the International Conference on the Applications of the Mössbauer Effect (ICAME 2017), Saint-Petersburg, Russia, 3–8 September 2017
Edited by Valentin Semenov
Rights and permissions
About this article
Cite this article
Müller, C.S., Auerbach, H., Stegmaier, K. et al. Mössbauer spectroscopy and DFT calculations on all protonation states of the 2Fe-2S cluster of the Rieske protein. Hyperfine Interact 238, 102 (2017). https://doi.org/10.1007/s10751-017-1471-1
Published:
DOI: https://doi.org/10.1007/s10751-017-1471-1