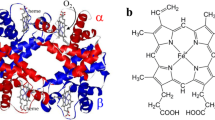
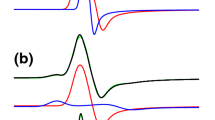
Abstract
Electron paramagnetic resonance (EPR) spectroscopy is performed on NO⋅-ligated hemo- and myoglobin at temperatures between 5 and 300 K. Apart from the standard X-band (9.5 GHz), data are taken at K-(24 GHz), Q-(34 GHz), W-(94 GHz) and Y-band (285 GHz) frequencies, respectively. The spectra are delineated into contributions of two EPR signatures differing ing-tensor symmetry, an axial and a rhombic one. The state contributions are found to differ with temperature and the states show temperature-dependentg-factor variations. In MbNO the axial species (denoted state II) is the only species observed at 300 K with X-band. Analysis of its development with decreasing temperature shows a partial transformation into a rhombic state (I). For this, at an intermediate temperature range (240–100 K), irregular line shapes are apparent, indicating disordered geometries of the Fe-NO-heme group. Regular rhombic lineshapes develop increasingly at temperatures below about 70 K. At 5–10 K, the rhombic state I dominates the EPR spectra but state II is still present. A similar development of spectra is observed in HbNO, for which, however, a rhombic contribution is already present at 300 K. Subunit-associated variations in state contributions and EPR appearance are not resolved at any frequency. Also, lineshape irregularities with decreasing temperature are not manifest in HbNO. These findings are used to present a structure-based picture of states I and II and their transformations and to discuss the origin of the line shape irregularities in MbNO.
Similar content being viewed by others
References
Henry Y.A., Guissani A., Ducastel B.: Nitric Oxide Research from Chemistry to Biology: EPR Spectroscopy of Nitrosylated Compounds. Austin, Tex.: R.G.Landes, Berlin: Springer (1996)
Perutz M.F., Kilmartin J.V., Nagai K., Szabo A., Simon S.R.: Biochemistry15, 378–387 (1976)
Maxwell J.C., Caughey W.C.: Biochemistry15, 388–396 (1976)
Henry Y., Banerjee R.: J. Mol. Biol.73, 469–482 (1973)
Cassoly R.: J. Mol. Biol.98, 581–595 (1975)
Ignarro L.J.: Annu. Rev. Pharmacol. Toxicol.30, 535 (1990)
Butler R.B., Williams D.L.H.: Chem. Soc. Rev.22, 233 (1993)
Lancaster J.R. Jr.: Am. Sci.80, 248 (1992)
Snyder S.H., Bredt D.S.: Sci. Am.266, 68–71 (1992)
Kerwin J.F. Jr., Lancaster J.R. Jr., Feldmann P.L.: J. Med. Chem,38, 4343–4362 (1995)
Griffith O.W., Stuehr D.J.: Annu. Rev. Physiol.57, 707 (1995)
Henry Y., Lepoivre M., Drapier J.-C., Ducroq C., Boucher J.-L., Guissani A.: FASEB J.7, 1124–1143 (1993)
Felisch M., Stamler J.S. (eds.): Methods in Nitric Oxide Research. Chichester: Wiley 1996.
Shiga T., Hwang K.-J., Tyuma I.: Biochemistry8, 378–383 (1969)
Morse R.H., Chan S.I.: J. Biol. Chem.255, 7876–7882 (1980)
Höhn M., Hüttermann J., Chien J.C.W., Dickinson L.C.: J. Am. Chem. Soc.105, 109–115 (1983)
Deatherage J.F., Moffat K.: J. Mol. Biol.134, 401–417 (1979)
Rein H., Ristau O., Scheler W.: FEBS Lett.24, 24–26 (1972)
Nagai K., Hori H., Yoshida S., Sakamoto H., Rokimoto H.: Biochim. Biophys. Acta532, 17–28 (1978)
Hüttermann J., Burgard C., Kappl R.: J. Chem. Soc. Faraday Trans.90, 3077–3087 (1994)
Kappl R., Hüttermann J.: Isr. J. Chem.29, 73–84 (1989)
Dickinson L.C., Symons M.C.R.: Chem. Soc. Rev.12, 387 (1984)
Wajnberg E., Linhares M.P., El-Jaick L.J., Bemski G.: Eur. Biophys. J.21, 57–61 (1992)
Flores M., Wajnberg E., Bemski G.: Biophys. J.78, 2107–2115 (2000)
Flores M., Wajnberg E., Bemski G.: Biophys. J.73, 3225–3229 (1997)
Tyryshkin A.M., Dikanov S.A., Reiijerse E.J., Burgard C., Hüttermann J.: J. Am. Chem. Soc.121, 3396–3406 (1999)
Wajnberg E., Bemski G., El-Jaick L.J., Alves O.C.: Int. J. Biol. Macromol.18, 231–235 (1996)
Hori H., Ikeda-Saito M., Yonetani T.: J. Biol. Chem.256, 7849–7855 (1981)
Magliozzo R.S., McCracken J., Peisach J.: Biochemistry26, 7923–7931 (1987)
Patchkovskii S., Ziegler T.: Inorg. Chem.39, 5334–5364 (2000)
Weiland B., Hüttermann J.: Int. J. Radiat. Biol.74, 341–358 (1998)
Eloranta J.: ftp://epr.chem.jyu.fi/pub/xemr (2001)
Riederer H., Hüttermann J., Boon P., Symons M.C.R.: J. Magn. Reson.54, 54–66 (1983)
Brucker E.A., Olson J.S., Ikeda-Saito M., Phillips G.N.: Proteins Struct. Funct. Genet.30, 352–356 (1998)
Chan N.L., Rogers P.H., Arnone A.: Biochemistry37, 16459–16464 (1998)
Yonetani T., Yamamoto H., Erman J.E., Leigh J.S. Jr., Reed C.H.: J. Biol. Chem.247, 2447–2455 (1972)
Overkamp M., Twilfer H., Gersonde K.: Z. Naturforsch.31c, 524–533 (1976)
Vanin A.F.: Biochemistry (Moscow)63, 782–793 (1998)
Stamler J.S.: Cell78, 931–936 (1994)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmidt, P.P., Kappl, R. & Hüttermann, J. On the mode of hexacoordinated NO-binding to myo- and hemoglobin: Variable-temperature EPR studies at multiple microwave frequencies. Appl. Magn. Reson. 21, 423–440 (2001). https://doi.org/10.1007/BF03162418
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03162418