Summary
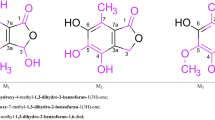
The absorption spectra of dibenzanthrone, 16: 17-dihydroxydibenzanthrone, 16: 17-and 3: 12-dimethoxydibenzanthrone have been determined ino-chlorophenol. The bathochromic shift of the dibenzanthrone spectrum on substitution of two methoxyl groups in the 16: 17-positions of dibenzanthrone is ascribed to the over-riding tendency of the dibenzanthrone ring system to remain planar and hence force the methoxyl groups also to be coplanar with the ring system.
The absorption spectra of 3:3′- and 4:4′-dibenzanthronyls have been reported and discussed.
Similar content being viewed by others
References
Brookeret al., Chem. Revs., 1947,41, 325;cf. Ferguson,Ibid., 1948,43, 385; Braudeet al., J. Chem. Soc., 1949, 1890.
Remick,Electronic Interpretations of Organic Chemistry, Wiley, New York, 2nd Ed., 1949, p. 323.
J. Am. Chem. Soc., 1940,62, 2906.
Ibid., 1945,67, 1838;cf. Grammaticakis,Bull. Soc. Chim., 1949,134, 761.
J. Am. Chem. Soc., 1949,71, 1714.
Picketet al., Ibid., 1936,58, 2296; 1950, 72, 44.
Ley and Pfeiffer,Ber., 1921,54, 363.
Calvin,J. Org. Chem., 1939,4, 256; Sherwood and CalvinJ. Am. Chem. Soc., 1942,64, 1350; see also Lewis and Calvin,Chem. Revs., 1939,25, 273.
Pestemer and Mayer-Pitsch,Monatsh., 1937,70, 104.
Twielacker and Ozegowski,Ber., 1940,73, 893.
Rodebushet al., J. Am. Chem. Soc., 1940,62, 2906; 1941,63, 3019; 1946,68, 896.
Westheimer and Mayer,J. Chem. Phys., 1946,14, 733; Hill,Ibid., 465.
Brode and Morris,J. Am. Chem. Soc., 1948,70, 2485;J. Org. Chem., 1948,13, 207.
Blout and Gofstein,J. Am. Chem. Soc., 1945,67, 13; see also Leonardet al., Ibid., 1950,72, 484, 5388.
Ibid., 1948,70, 199.
Jones, Ibid., 1941,63, 1658; 1945,67, 2127; Hirshberg,Trans. Faraday Soc., 1948,44, 285.
Z. physik. Chem., 1951,155A, 353;cf. Alberman,J. Chem. Soc., 1952, 3284; Beale and Roe,J. Amer. Chem. Soc., 1952,74, 2302.
Robertson,Proc. Roy. Soc., 1935,150A, 348.
See also Weigand and Merkel,Ann., 1947,557, 242.
Cook, Jones and Polya,J. Chem. Soc., 1939, 1315.
Zechmeister,Chem. Revs., 1944,34, 267.
J. Am. Chem. Soc., 1942,64, 593.
Jones, Ibid., 1943,65, 1815.
Ibid., 1941,63, 3252.
Jones, Ibid., 1941,63, 313; see also Jones,Chem. Revs., 1943.32, 1.
J. Am. Chem. Soc., 1940,62, 2295.
Friedel and Orchin,Ultraviolet spectra of Aromatic Compounds, Wiley, New York, 1951.
P. N. Pandit,Ph.D. Thesis, University of Bombay, 1952.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Padhye, M.R., Rao, N.R. & Venkataraman, K. Anthraquinone and anthrone series. Proc. Indian Acad. Sci. (Math. Sci.) 38, 307–319 (1953). https://doi.org/10.1007/BF03045259
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03045259