Abstract
Duett™, a novel vascular sealing device, was first clinically used in July 1997. A European multi-center registry was established to evaluate the safety and procedural success of the Duett™ sealing device in a broad range of patients undergoing diagnostic or interventional endovascular procedures.
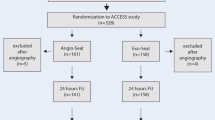
At 25 European sites 1587 patients were enrolled. All patients (≥ 18 years) must have given informed consent for the use of the sealing device after a diagnostic and/or interventional endovascular procedure performed via a femoral arterial approach. Standard length (<-10 cm) 5 to 9 F introducer sheaths had to be used. An ACT of ≤400 s, and any approved GP IIb/IIIa platelet receptor antagonist was permitted.
Successful deployment could be achieved in 96.2% (1526/1587 patients) with complete hemostasis within 2 to 5 minutes in over 95% of the patients. The complication-free rate was 96.4%. Arterial occlusions were rare (4 patients) and successfully treated with surgical repair in 1 and with thrombolysis in 3 patients. Pseudoaneurysms occurred in 34 patients, the majority (30/34) were successfully treated with ultrasound-guided compression or resolved spontaneously. The total rate of major complications was 2.6% (41/1587).
The final results of the European registry demonstrate that the Duett™ sealing device can be used with a high procedural success following diagnostic and interventional endovascular procedures. The incidence of major complications is low and comparable to all other approved vascular closure devices and manual compression. CE-mark certification was approved at the end of 1998.
Zusammenfassung
Duett™, ein neues arterielles Verschlußsystem, wurde zum ersten Mal im Juli 1997 klinisch eingesetzt. Um die Sicherheit und Wirksamkeit bei einem breiten Spektrum von Patienten nach diagnostischem bzw. interventionellem Katheter zu beurteilen, wurde ein Europäisches Multicenterregister eingerichtet.
An 25 europäischen Zentren wurden 1587 Patienten in das Register aufgenommen. Alle Patienten (≥18 Jahre) mußten sich mit der Anwendung des Verschlußsystems (femoraler Zugang) einverstanden erklären. Bei der Katheteruntersuchung sollte ein Schleusendurchmesser von 5 bis 9 F bei einer Standardlänge bis zu 10 cm verwendet werden. Die ACT sollte nicht über 400 s betragen, die zusätzliche Gabe eines zugelassenen GP-IIb/IIIa-Rezeptorantagonisten war gestattet.
Das Verschlußsystem konnte in 96,2% (1526/1587 Patienten) erfolgreich eingesetzt werden, eine vollständige Hämostase trat innerhalb von zwei bis fünf Minuten bei über 95% der Patienten ein. Bei 96,4% der Patienten wurden keine Komplikationen beobachtet. Ein intraluminaler Verschluß der Femoralarterie war selten und wurde erfolgreich operativ bei cinem und mittels Thrombolyse bei drei Patienten behandelt. Beidrei Patienten war eine Bluttransfusion erforderlich. 30 der 34 beobachteten Pseudoaneurysmen konnten ultraschallgestützt erfolgreich komprimiert werden oder bildeten sich spontan zurück. Da entsprechend der FDA-Definition auch diese Pseudoaneurysmen als größere Komplikation klassifiziert werden müssen, errechnete sich eine Gesamtrate an größeren Komplikationen von 2,6% (41/1587).
Die endgültigen Ergebnisse des Europäischen Multicenter-registers zeigen, daß das Deutt™-Verschlußsystem sicher urfolgreich nach diagnostischer und interventioneller Katheteruntersuchung eingesetzt werden kann. Die Rate an größeren Komplikationen ist mit der aller anderen zugelassenen arteriellen Verschlußsysteme sowie der manuellen Kompression vergleichbar. Die CE-Zertifizierung wurde Ende 1998 erteilt.
Similar content being viewed by others
References
Baim DS, Pinkerton CA, Schatz RA, et al. Acute results of the STAND-II percutaneous vascular surgical device trial. Circulation 1997;96:I-442.abstract.
Bartorelli AL, Sganzerla P, Fabbiocchi F, et al. Prompt and safe femoral hemostasis with a collagen device after intracoronary implantation of Palmaz Schatz stents. Am Heart J 1995;130:26–32.
Brachmann J, Ansah M, Kosinski EJ, et al. Improved clinical effectiveness with a collagen vascular hemostasis device for shortened immobilization time following diagnostic angiography and percutaneous transluminal coronary angioplasty. Am J Cardiol 1998;81:1502–5.
Carete RG, Webb JG, Miyagishima R, et al. Groin complications associated with collagen plug closure of femoral arterial puncture sites in anticoagulated patients. Cathet Cardiovasc Diagn 1998;43:124–9.
Chamberlin JR, Lardi AB, McKeever LS, et al. Use of vascular sealing devices (VasoSeal and Perclose) versus assisted manual compression (Femostop) in transcatheter coronary interventions requiring abciximab (ReoPro). Cathet Cardiovasc Intervent 1999;47:143–47.
David WJ, Grullon CP, Tillman SB. Clinical and cost outcomes after implementation of suture mediated closure device as standard of care for outpatient diagnostic procedures. Am J Cardiol 1999;84:Suppl 6A:52P.
Ellis SG, Mooney M, Talley JD, et al. DUETT femoral artery closure device vs manual compression after diagnostic or interventional catheterization: Results of the SEAL trial. Circulation 1999;100:I-513.
Ernst S, Tjonjoegin RM, Schräder R, et al. Immediate sealing of arterial puncture sites after cardiac catheterization and coronary angioplasty using a biodegradable collagen plug: results of an international registry. J Am Coll Cardiol 1993;21:851–5.
Gerckens U, Cattelaens N, Lampe E-G, et al. Management of arterial puncture site after catheterization procedures: Evaluating a suture-mediated closure device. Am J Cardiol 1999;83:1658–63.
Gershony G, Brock JM, Powell JS. A novel vascular sealing device for closure of percutaneous vascular access sites. Cathet Cardiovasc Diagn 1998;45:82–8.
Heyer G, Atzenhofer K, Meixl H, et al. Results in patients treated with the Duett™ vascular sealing device following diagnostic catheterization and PTCA. Am J Cardiol 1999;84:Suppl 6A:55P.
Kussmaul WG 3rd, Buchbinder M, Whitlow PL, et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J Am Coll Cardiol 1995;25:1685–92.
Loubeyre C, Karam C, Fajadet J, et al. Prospective use of a percutaneous vascular closure device in patients undergoing PTCA and stenting. J Am Coll Cardiol 1997;29:278-A.abstract.
Lunney L, Karim K, Little T. Vasoseal hemostasis following coronary interventions with abciximab. Cathet Cardiovasc Diagn 1998;44:405–6.
Mooney MR, Ellis SE, Yehyawi KJ, et al. Immediate sealing of arterial puncture sites after cardiac catheterization and coronary interventions: Initial U. S. Feasibility trial using the DUETT vascular closure device. Circulation 1998;98:I-88.abstract.
Morice MC, Lefevre T. Immediate post PTCA percutaneous suture of femoral arteries with the Perclose device: results of high volume users. J Am Coll Cardiol 1998;31:101A.abstract.
Sanborn TA, Gibbs HH, Brinker JA, et al. A multicenter randomized trial comparing a percutaneous collagen hemostasis device with conventional manual compression after diagnostic angiography and angioplasty. J Am Coll Cardiol 1993;22:1273–9.
Schräder R, Steinbacher S, Burger W, et al. Collagen application for sealing of arterial puncture sites in comparison to pressure dressing: A randomized trial. Cathet Cardiovasc Diagn 1992;27:298–302.
Schwarten DE, Pinkerton CA, Vetter JW, et al. Acute results of the STAND-I percutaneous vascular surgical device trial. Circulation 1997;96:I-137.abstract.
Silber S, Björvik A, Mühling H, et al. Usefulness of collagen plugging with VasoSeal® after PTCA as compared to manual compression with identical sheath dwell times. Cathet Cardiovasc Diagn 1998;43:421–7.
Silber S, Dörr R, Mühling H, et al. Sheath pulling immediately after PTCA: Comparison of two different deployment techniques for the hemostatic puncture closure device. A prospective, randomized study. Cathet Cardiovasc Diagn 1997;41:378–83.
Silber S, Gershony G, Schön B, et al. A novel vascular sealing device for closure of percutaneous arterial access sites. Am J Cardiol 1999;83:1248–52.
Silber S, Schön N, Seidel N, et al. Akzidenteller Verschluß einer A. femoralis communis nach Angio-Seal™-Applikation. Z Kardiol 1998:87:51–5.
Silber S. Hemostasis success rates and local complications with collagen after femoral access for cardiac catheterization: Analysis of 6007 published patients. Am Heart J 1998;135:152–6.
Silber S. Rapid hemostasis of arterial puncture sites with collagen in patients undergoing diagnostic and interventional cardiac catheterization. Clin Cardiol 1997;20:981–92.
Turi ZG. Plugging the artery with a suspension: A cautious appraisal. Cathet Cardiovasc Diagn 1998;45:90–1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silber, S., Tofte, A.J., Kjellevand, T.O. et al. Final report of the European multi-center registry using the Deutt™ vascular sealing device. Herz 24, 620–623 (1999). https://doi.org/10.1007/BF03044486
Issue Date:
DOI: https://doi.org/10.1007/BF03044486