Abstract
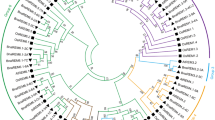
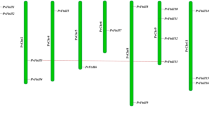
Two adjacent polygalacturonase-inhibiting protein (PGIP) genes were characterized from the model legumeMedicago truncatula. MtPGIP1 andMtPGIP2 were isolated from a single bacterial artificial chromosome clone identified from library-screening with cDNA probes. Ten and nine characteristic stretches of leucine-rich repeats, respectively, were identified from the predicted MtPCIP1 and MtPGIP2, showing 58% sequence identity at the amino acid level. TheseMtPGIP genes are likely present as a small gene family. Transcripts encoding MtPGIP1 were expressed highly in the flowers and at low levels in the roots and stems, whereas those encoding MtPGIP2 were not detected in any untreated organs. Inoculation of theM. truncatula cultivar “Jemalong” with the pathogenic fungusColletotrichum trifolii induced a hypersensitive response and the expression of both genes. The two genes were also expressed in response to the application of jasmonic acid, although mechanical wounding induced onlyMtPGIP1 and salicylic acid induced neither. Abiotic stresses, such as high-salt, cold, or drought, induced the expression ofMtPGIP1, whereas low-temperature stress inducedMtPGIP1 only. Consistent with these observations, sequence elements specific to plant defense and stress responses were identified, in varying numbers, from the putative promoter regions of the two genes. Furthermore, supportive of their putative functional roles, bacterially expressed recombinant MtPGIP1 and MtPGIP2 inhibited fungal polygalacturonase activity. Therefore, these results suggest thatMtPGIP1 andMtPGIP2, copies that presumably arose from duplication, are regulated by separate signaling pathways and likely play roles in response to pathogenic and environmental stresses.
Similar content being viewed by others
Literature Cited
Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K (1997) Role ofArabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell9: 1859–1868
Albrecht C, Geurts R, Bisseling T (1999) Legume nodulation and mycorrhizae formation; two extremes in host specificity meet. EMBO J18: 281–288
Baker SS, Wilhelm KS, Thomashow MF (1994) The 5-regionof Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol24: 701–713
Barker DG, Bianchi S, Blondon F, Dattee FY, Duc G, Essad S, Flament P, Gallusci P, Genier G, Guy P, Muel X, Tourneur J, Denarie J, Huguet T (1990)Medicago truncatula, a model plant for studying the molecular genetics of theSinorhizobium-legume symbiosis. Plant Mol Biol Rep8: 40–49
Blondon F, Marie D, Brown S, Kondorosi A (1994) Genome size and base composition inMedicago sativa andM. truncatula species. Genome37: 264–270
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem72: 248–254
Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM (2003) A role for the GCC-box in jasmonate-mediated activation of thePDF1.2 geneof Arabidopsis. Plant Physiol132: 1020–1032
Cannon SB, Crow JA, Heuer ML, Wang X, Cannon EK, Dwan C, Lamblin AF, Vasdewani J, Mudge J, Cook A, Gish J, Cheung F, Kenton S, Kunau TM, Brown D, May GD, Kim D, Cook DR, Roe BA, Town CD, Young ND, Retzel EF (2005) Databases and information integration for theMedicago truncatula genome and transcriptome. Plant Physiol138: 38–46
Cullimore J, Denarie J (2003) How legumes select their sweet talking symbionts. Science302: 575–578
de Lorenzo G, D’Ovidio R, Cervone F (2001) The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol39: 313–335
Desiderio A, Aracri B, Leckie F, Mattei B, Salvi G, Tigelaar H, van Roekel JS, Baulcombe DC, Melchers LS, de Lorenzo G, Cervone F (1997) Polygalacturonase-inhibiting proteins (PGIPs) with different specificities are expressed inPhaseolus vulgaris. Mol Plant Microbe Interact10: 852–860
Doares SH, Syrovets T, Weiler EW, Ryan CA (1995) Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA92: 4095–4098
D’Ovidio R, Raiola A, Capodicasa C, Devoto A, Pontiggia D, Roberti S, Galletti R, Conti E, O’Sullivan D, de Lorenzo G (2004) Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiol135: 2424–2435
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci5: 199–206
Favaron F, Destro T, D’Ovidio R (2000) Transcript accumulation of polygalacturonase inhibiting protein (PGIP) following pathogen infection in soybean. J Plant Pathol82: 103–109
Federici L, Caprari C, Mattei B, Savino C, Di Matteo A, de Lorenzo G, Cervone F, Tsernoglou D (2001) Structural requirements of endopolygalacturonase for the interaction with PGIP (polygalacturonase-inhibiting protein). Proc Natl Acad Sci USA98: 13425–13430
Ferrari S, Vairo D, Ausubel F, Cervone F, de Lorenzo G (2003) Tandemly duplicatedArabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell15: 93–106
Gamas P, De Billy F, Truchet G (1998) Symbiosis-specific expression of twoMedicago truncatula nodulin genes,MtN1 andMtN13, encoding products homologous to plant defense proteins. Mol Plant Microbe Interact11: 393–403
Handberg K, Stougaard J (1992)Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J2: 487–496
Higgins DG, Sharp PM (1988) CLUTSTAL: A package for performing multiple sequence alignment on microcomputer. Gene73: 237–244
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: Evolution and utilization. Annu Rev Phytopathol39: 285–312
Jang S, Lee B, Kim C, Kim S-J, Yim J, Han J-J, Lee S, Kim SS, An G (2003) TheOsFOR1 gene encodes a polygalacturonase-inhibiting protein (PGIP) that regulates floral organ number in rice. Plant Mol Biol53: 357–369
Jin H, Martin C (2000) Multifunctionality and diversity within the plant MYB-gene family. Nucleic Acids Res28: 2004–2011
Kim J-Y, Lee S-C, Jung K-H, An G, Kim S-R (2004) Characterization of a cold-responsive gene,OsPTR1, isolated from the screening of ß-glucuronidase(GUS) genetrapped rice. J Plant Biol47: 133–141
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature227: 680–685
Lee H, Kim H, An CS (2004) Cloning and expression analysis of 2-on-2 hemoglobin from soybean. J Plant Biol47: 92–98
Lenne JM (1992)Colletotrichum diseases of legumes,In Bailey JA, jeger MJ, eds,Colletotrichum: Biology, Pathology and Control. CAB International, Wallingford, pp 134–166
Li R, Rimmer R, Yu M, Sharpe AG, Seguin-Swartz G, Lydiate D, Hegedus DD (2003) TwoBrassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta217: 299–308
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, inArabidopsis. Plant Cell10: 1391–1406
Mahalingam R, Gomez-Buitrago A, Eckardt N, Shah N, Guevara-Garcia A, Day P, Raina R, Fedoroff NV (2003) Characterizing the stress/defense transcriptome ofArabidopsis. Genome Biol4: R20
Martin GB, Bogdanove AJ, Sessa G (2003) Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol54: 23–61
Nam YW, Penmetsa RV, Endre G, Uribe P, Kim D, Cook DR (1999) Construction of a bacterial artificial chromosome library ofMedicago truncatula and identification of clones containing ethylene-response genes. Theor Appl Genet98: 638–646
Park S-Y, Nam Y-W (2006) Construction of a bacterial artificial chromosome library containing largeBamHI genomic fragments ofMedicago truncatula and identification of genes linked to hyper-nodulating genes. J Microbiol Biotechnol (in press)
Pieterse CMJ, van Loon LC (1999) Salicylic acid-independent plant defense pathways. Trends Plant Sci4: 52–58
Pontier D, Balague C, Bezombes-Marion I, Tronchet M, Deslandes L, Roby D (2001) Identification of a novel pathogen-responsive element in promoter of the tobacco gene HSR203J, a molecular marker of the hypersensitive response. Plant J26: 495–507
Powell AL, van Kan J, ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact13: 942–950
Rogers SO, Bendich AJ (1994) Extraction of total cellular DNA from plants, algae, and fungi,In Gelvin SB, Schilperoort RA, eds, Plant Molecular Biology Manual D1. Kluwer Academic Publishers, Dordrecht, pp 1–8
Rombauts S, Dehais P, van Montagu M, Rouze P (1999) PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res27: 295–296
Rushton PJ, Somssich IE (1998) Transcriptional control of plant genes responsive to pathogens. Curr Opin Plant Biol1: 311–315
Surette MG, Stock JB (1996) Role of α-helical coiled-coil interactions in receptor dimerization, signaling, and adaptation during bacterial chemotaxis. J Biol Chem271: 17966–17973
Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways inArabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA95: 15107–15111
Torregrosa C, Cluzet S, Fournier J, Huguet T, Gamas P, Prosperi J-M, Esquerre-Tugaye M-T, Dumas B, Jacquet C (2004) Cytological, genetic, and molecular analysis to characterize compatible and incompatible interactions betweenMedicago truncatula andColletotrichum trifolii. Mol Plant Microbe Interact17: 909–920
Toubart P, Desiderio A, Salvi G, Cervone F, Daroda L, de Lorenzo G, Bergmann C, Darvill AG, Albersheim P (1992) Cloning and characterization of the gene encoding the endopolygalacturonase-inhibiting protein (PGIP) ofPhaseolus vulgaris L. Plant J2: 367–373
Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Men-doza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, Weigel D, Harrison MJ (2000) Transformation ofMedicago truncatula via infiltration of seedlings or flowering plants withAgrobacterium. Plant J22: 531–541
VandenBosch KA, Stacey G (2003) Summaries of legume genomics projects from around the globe. Community resources for crops and models. Plant Physiol131: 840–865
Welin BV, Olson A, Nylander M, Palva ET (1994) Characterization and differential expression ofdhn/lea/rab-like genes during cold acclimation and drought stress inArabidopsis thaliana. Plant Mol Biol26: 131–144
Xu B, Timko M (2004) Methyl jasmonate induced expression of the tobacco putrescine N-methyltransferase genes requires both G-box and GCC-motif elements. Plant Mol Biol55: 743–761
Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting. regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci10: 88–94
Yao CL, Conway WS, Ren RH, Smith D, Ross GS, Sams CE (1999) Gene encoding polygalacturonase inhibitor in apple fruit is developmentally regulated and activated by wounding and fungal infection. Plant Mol Biol39: 1231–1241
York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P (1985) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol118: 3–40
Zarka DG, Vogel JT, Cook D, Thomashow MF (2003) Cold induction ofArabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol133: 910–918
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, KH., Nam, YW. Genomic organization and differential expression of two polygalacturonase-inhibiting protein genes fromMedicago truncatula . J. Plant Biol. 48, 467–478 (2005). https://doi.org/10.1007/BF03030589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030589