Abstract
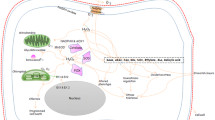
The oxidative burst, the rapid production of O2- and H2O2 by plant cells in response to pathogens and Stressors, is a critical step in plant disease resistance and is controlled by several different elicitor-initiated signaling pathways. While different defense elicitors appear to activate disparate initial steps in signaling the oxidative burst, all of the elicitors tested thus far appear to stimulate pathways that converge on the same three core signaling intermediates: 1) the Ca2+-independent activation of a mitogen-activated protein kinase (MAPK) family member, 2) the influx of Ca2+ into the cytosol, deriving most critically from an internal compartment, and 3) the Ca2+-dependent activation of additional protein kinases including a second MAPK homologue and possibly calcium dependent protein kinases (CDPKs). Data from several recent reports are summarized to place these signaling events into a complete and updated model of signaling to the plant oxidative burst.
Similar content being viewed by others
Abbreviations
- CDPK:
-
Calcium Dependent Protein Kinase
- GMK1:
-
Glycine max Mitogen Activated Protein Kinase 1
- GMK2:
-
Glycine max Mitogen Activated Protein Kinase 2
- MAPK:
-
Mitogen Activated Protein Kinase
- SIPK:
-
Salicylic Acid-Induced Protein Kinase
- WIPK:
-
Wounding-lnduced Protein Kinase
Literature cited
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Ann Rev Phytopath33: 299–321
Blumwald E, Aharon GS, Lam BCH (1998) Early signal transduction pathways in plantpathogen interactions. Trends Plant Sci3: 342–346
Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P (1999) Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radic Res SuppI: SI37–145
Bolwell GP, Daview DR, Gerrish C, Auh C-Y, Murphy TM (1998) Comparative biochemistry of the oxidative burst produced by rose and french bean cells reveals two distinct mechanisms. Plant Physiol116: 1379–1385.
Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A (1999) Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem274: 34699–34705
Bowler C, Fluhr R (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci5: 241–246
Cazale A-C, Droillard M-J, Wilson C, Heberle-Bors H, Barbier-Brygoo H, Lauriere C (1999) MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: Towards the oxidative burst response. Plant J19: 297–307
Cessna SG, Chandra S, Low PS (1998) The hypo-osmotic shock activated Ca2+ transient in suspension cultured tobacco cells is the product of external and internal cellular Ca2+ sources. J Biol Chem273: 27286–27291.
Cessna SG, Low PS (2001a) Activation of the oxidative burst in aequorin-transformed Nicotiana tabacum cells is mediated by protein kinase- and anion channel-dependent release of Ca2+ from internal stores. Planta214: 126–134
Cessna SG, Low PS (2001 b) An apoplastic Ca2+ sensor regulates internal Ca2+ release in aequorin-transformed tobacco cells. J Biol Chem276: 10655–10662
Chandra S, Low PS (1995) Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA92: 4120–4123
Chandra S, Heinstein PF, andLow PS (1996a) Activation of phospholipase A by plant defense elicitors. Plant Physiol110: 979–986
Chandra S, Martin GB, Low PS (1996b) The Pto kinase mediates a signaling pathway leading to the oxidative burst in tomato. Proc Natl Acad Sci USA93: 13393–13397
Chandra S, Stennis M, Low PS (1997) Measurement of Ca2+ fluxes during dictation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem272: 28274–28280
Clapham DE (1995) Calcium signaling. Cell80: 259–268
Dwyer SC, Legendre L, Low PS, Leto TL (1996) Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta1289: 231–237
Farmer EE, Pearce G, Ryan CA (1989) In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci USA86: 539–542
Felix G, Grosskopf DG, Regenass M, Boiler T (1991) Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA88: 8831–8834
Felix G, Regenass M, Spanu P, Boiler T (1994) The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [32P] phosphate. Proc Natl Acad Sci USA91: 952–956
Gelli A, Blumwald E (1997) Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J Membr Biol155: 35–45
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol124: 21–30
Gupta R, Luan S (2003) Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol132: 1149–1152
Hoyos ME, Zhang S (2000) Calcium-independent activation of salicylic acid-induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol122: 1355–1363
Huang Y, Li H, Gupta R, Morris PC, Luan S, Kieber JJ (2000) ATMPK4, an Arabidopsis homolog of mitogen-activated protein kinase, is activatedin vitro by AtMEKI through threonine phopshorylation. Plant Physiol122: 1301–1310
Jabs T, Schope M, Collig C, Hahlbrock K, Scheel D (1997) Elicitor stimulated ion fluxes and O2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA94: 4800–4805
Jonak C, Okresz L, Bogre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol5: 415–424
ba]Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein withCa 2+ binding motifs. Plant Cell10: 255–266
Kim J (2002) Ph. D. thesis, Dept. of Chemistry, Purdue University, IN, USA
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature352: 524–526
Kovtun Y, Chiu VV-L, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA97: 29402945
Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors inNicotiana plumbaginifolia cells. Plant Cell14: 2627–2641
Lee J, Klessig DF, Nurberger T (2001) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell13: 1079–1093
Legendre L, Heinstein PF, Low PS (1992) Evidence for participation of GTP-binding proteins in the elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem267: 20140–20147
Legendre L, Yueh YG, Grain R, Haddock N, Heinstein PG, Low PS (1993) Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem268: 24559–24563
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell79: 583–593
Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D (1997) Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science276: 2054–2057
Liu J, Zhu JK (1998) A calcineurin sensor homolog required for plant salt tolerance. Science280: 1943–1945
Matthieu Y, Sanchez RJ, Droillard MJ, Lapous D, Lauriere C, Guern J (1996) Involvement of protein phosphorylation in the early steps of transduction of the oligogalac-turonide signal in tobacco cells. Plant Physiol Biochem34: 399–408
Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak S-S, Kim DH, Nam J, Bahk J, Hong JC, Lee SY, Cho MJ, Lim CO, Yun D-J (2003) NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulat cellular reclox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA100: 358–363
Navazio L, Moscatielio R, Bellincampi D, Baldan B, Meggio F, Brini M, Bowler C, Mariani P (2002) The role of calcium in oligogalacturonide-activated signaling in soybean cells. Planta215: 596–605
Nuhse TS, Peck SC, Hirt H, Boiler T (2000) Microbial elicitors induce activation and dual phosphorylation of theArabidopsis thaliana MAPK 6. J Biol Chem275: 7521–7526
Olmos E, Martinez-Solano JR, Piqueras A, Hellin E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot54: 291–301
Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemicaily in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA96: 6553–6557
Peck SC, Nuhse TS, Hess D, Iglesias A, Meins F, Boiler T (2001) Directed proteomics identifies a plant-specific protein rapidly phosphorylated in response to bacterial and fungal elicitors. Plant Cell13: 1467–1475
Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johanson B, Nielsen HB, Lacy M, Austin J, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J (2000)Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell103: 1111–1120
Reymond P, Grunberger S, Paul K, Muller M, Farmer EE (1995) Oligogalacturonide defense signals in plants: large fragments interact with the plasma membrane in vitro. Proc Natl Acad Sci USA92: 4145–4149
Romeis T, Ludwig AA, Martin R, Jones JDG (2001) Calcium-dependent protein kinases play and essential role in a plant defence response. EMBO J20: 5556–5567
Romeis T, Piedras P, Jones JD (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell12: 803–816.
Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG (1999) Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound and salicylate responses. Plant Cell11: 273–287
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91 (phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol126: 1281–1290
Samuel MA, Ellis BE (2002) Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell14: 2059–2069
Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell11: 691–706
Schwake R, Hager A (1992) Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein kinase activity. Planta187: 136–141
Scofield SR, Tobias CM, Rathjen JP, Chang JH, Lavelle DT, Michelmore RW, Staskawicz BJ (1996) Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science274: 2063–2065
Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science270: 1988–1992
Tang X, Frederick RD, Zhou J, Halterman DA, Jia Y, Martin GB (1996) Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science274: 2060–2063
Tavernier E, Wendehenne D, Blein JP, Pugin A (1995) Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol109: 1025–1031
Taylor AT, Kim J, Low PS (2001) Involvement of mitogenactivated protein kinase activation in the signal-transduction pathways of the soya bean oxidative burst. Bio-chem J355(Pt 3): 795–803
Taylor ATS, Steffen N, Weitzel CS, Rheinhardt SJ, Johnson EG (2003) Characterization of the oxidative regulation of a phosphatase from Arabidopsis. Plant Journal (submitted)
Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91 phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA99: 517–522
Ward JM, Pei ZM, Schroeder Jl (1995) Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell7: 833–844
Wojtaszek B (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J322: 681–692
Xing T, Higgins VJ, Blumwald E (1997) Race-specific elicitors ofCladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Ceil9: 249–259
Yang K-Y, Liu Y, Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA98: 741–746
Zhang S, Klessig DF (1997) Salicylic acid activates a 48 kDa MAP kinase in tobacco. Plant Cell9: 809–824
Zhang S, Klessig DF (2000) Pathogen-induced MAP kinases in tobacco. Results Probl Cell Differ27: 65–84
Zhang S, Klessig DF (2001) MAPK cascades in plant defense signaling. Trends Plant Sci6: 520527
Zhang S, Liu Y, Klessig DF (2000) Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J23: 339–347
Zhou J, Loh YT, Bressan RA, Martin GB (1995) The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell83: 925–935
Zimmermann S, Nurnberger T, Frachisse JM, Wirtz W, Guern J, Hedrich R, Scheel D (1997) Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc Natl Acad Sci USA94:2751–2755
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cessna, S.G., Kim, J. & Taylor, A.T.S. Cytosolic Ca2+ pulses and protein kinase activation in the signal transduction pathways leading to the plant oxidative burst. J. Plant Biol. 46, 215–222 (2003). https://doi.org/10.1007/BF03030367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030367