Abstract
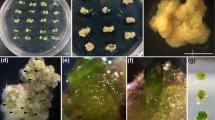
To maintain embryogenic cell lines ofPimpinella brachycarpa, we suspension-cultured friable and rapidly growing yellowish calli in an MS liquid medium containing 0.2 ~ 2,4-D and 0.5pM BAP. Efficient somatic embryogenesis was achieved when selected cells were then transferred to an MS medium (0.2% gelrite) that contained 0.2gM 2,4-D, 0.5 uM BAP, and 10.0 laM TDZ (thidiazuron). These cells were cultured at 27°C under continuous illumination (21.5 I~E m-2 s-l). Embryogenic calli expanded about four-fold, and developed into pale yellow calli. Somatic embryogenesis was initiated only from glossy and nodular-type calli. After two more weeks of culture, globular embryos appeared on the surface of calli grown in the MS medium that contained 10.0 /aM TDZ only, or in combination with 0.5 gM NAA. Experimenting with 2,4-D, an auxin, to promote embryogenic calli resulted in excessive browning and death. We overcame this problem by growing glossy embryogenic and nodular calli on media that contained 10.0 gM TDZ. Calli that were not treated with TDZ turned dark brown and were not viable. Up to 74% of the calli showed somatic embryos when the medium was supplemented with 10.0 uM TDZ and 0.5 uM NAA. Embryos from these TDZ-induced, somatic embryogenic calli grew efficiently, forming multiple shoots and developing into normal plants. Therefore, efficient differentiation of suspension-cultured cell clusters into embryogenic calli, along with treatment of subsequent somatic embryos by TDZ, suggests that TDZ probably helps in establishing the optimum cytokinin-auxin ratio required for induction and expression of somatic embryogenesis.
Similar content being viewed by others
Literature Cited
Aleith F, Richter G (1990) Gene expression during induction of somatic embryogenesis in carrot cell suspension. Planta183: 17–24
Binh O Q, Heszky LE (1990) Restoration of the regeneration potential of long term cell culture in rice(Oryza sativa) by salt pretreatment. Plant Physiol136: 336–340
Bueno MA, Astorgo R, Manzanera JA (1992) Plant regeneration through somatic embryogenesis inQuercus suber Physiol Plant85: 30–34
Chalupa V (1990) Plant regeneration by somatic embryogenesis from cultured immature embryos of oak(Quercus robur) and linden(Tilia cordata). Plant Cell Rep9: 398–401
Data SK, Data K, Potrycus I (1990) Embryogenesis and regeneration from microspores of both indica and japonica rice(Oryza sativa). Plant Sci67: 83–88
Feirer RP, Simon PW (1991) Biochemical differences between carrot inbreds differing in plant regeneration potential Plant Cell RepI0: 152–155
Franz G, Hatzopoulos P, Jones TJ, Kraus SM, Sung ZR (1989) Molecular and genetic analysis of an embryogenic gene DC8, fromDaucus carota L. Mol Gene Genet218: 143–151
Gmitter FG, Moore GA (1986) Plant regeneration from undeveloped ovules and embryogenic calli ofOtrus: Embryo production, germination and survival Rant Cell Tiss Org Cult6: 139–147
Litz RE, Gray DJ (1995) Somatic embryogenesis for agricultural improvement. World J Microbiol Biotechnol11: 416–425
Malik KA, Saxena PK (1992) Thidiazuron induces high fiequency shoot regeneration in intact seedlings of pea(Pisum sativum), chickpea(Cicer arietinum) and lentil(Lens culinaris). Aust J Plant Physiol19: 731–740
Moon HK, Yang Y, Lee SK (1994) Rapid micropropagation ofPimpinella brachycarpa via somatic embryogenesis. Korean J Plant Tiss Cult21: 85–90
Murty BNS, Murch S, Saxena PK (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut(Arachis hypogaea): Endogenous growth regulators levels and significance of cotyledons. Physiol Plant94: 268–276
Nabors MW, Heyser TA, Dykes Demott KJ (1983) Long duration high frequency of plant generation from cereal tissue culture. Pianta157: 385–389
Saxena PK, Malik KA, Gilt R (1992) Induction of thidiazuton of somatic embryogenesis in intact seedlings of peanut. Planta187: 421–424
Thomas JC, Katterman FR (1986) Cytokinin activity induced by thidiazuron. Plant Physio181: 681–683
Thorpe TA (1993) In vitro organogenesis and somatic embryogenesis: Physiological and biochemical aspects,In KA Roubelakis-Angelakis, K Tran Tranh Van, eds, Morphogenesis Plants, Plenum Press, New York, pp 19–38
Tremblay L, Tremblay FM (1991) Effect of gelling agents, ammonium nitrate, and light on the development ofPicea mariana andP/cea rubens somatic embryo. Plant Sci77: 233–242
van Schaik CE, Posthuma MJDJ, Jacobsen E (1996) Plant regeneration through somatic embryogenesis from callus induced on immature embryos of AIstroemeriaspp. L. Plant Cell Rep15: 377–380
Wang D, Wergin WP, Zimmerman RH (1984) Somatic embryogenesis and plant regeneration from immature embryos of strawberry. HortScience19: 71–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.C., Chang, M.Y., Son, S.I. et al. Thidiazuron required for efficient somatic embryogenesis from suspension-cultured cells ofPimpinella brachycarpa . J. Plant Biol. 44, 224–230 (2001). https://doi.org/10.1007/BF03030356
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03030356