Abstract
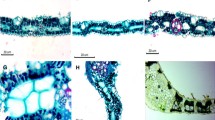
Structural differentiation of Kranz anatomy has been investigated in leaf cross sections of two C-4 Poaceae:Digitaria sanguinalis andSetaria viridis. The study mainly focused on cellular and interfacial features of bundle sheath (BS) and mesophyll (MS) cells of the C-4 structure. Prominent BS, spaced by only two MS cells apart, were surrounded concentrically by a layer of MS cells. BS cells ofS. viridis had centrifugally arranged relatively large chloroplasts containing much starch, but the chloroplasts had agrana to rudimentary grana. Structural and size dimorphisms, when starch was present, were detected between BS and MS chloroplasts. Loosely arranged MS cells had peripherally displaced smaller chloroplasts containing little to none starch. BS chloroplasts ofD. sanguinalis were similar to those ofS. viridis, but had very little starch and well-developed long agranal stroma lamella. Features of MS cells were similar in both species, but well-defined peripheral reticulum (PR) was easily recognized in MS chloroplasts ofS. viridis. Virtually no PR was developed in BS chloroplasts examined. BS cells contained more mitochondria and microbodies, but no structural dimorphism was noticed. The electron-dense suberized lamella were often observed between BS and MS cells, especially in the primary wall of BS cells. It was most frequently found at the BS and MS cell interfaces and terminated in radial walls of the adjacent BS cells. Prominent pits with plasmodesmata (pd) were seen in the walls of both cells. There also were numerous pd in outer tangential walls of the BS cells. The number of pd ranged from 20 to 60. The pd trasversed a segment of cell wall much thinner than the adjacent wall. The current cellular data have been compared to the ultrastructural features known in leaves of other C-4 plants, especially NADP-ME species.
Similar content being viewed by others
Literature Cited
Black, C.C. and H.H. Mollenhauer. 1971 Structure and distribution of chloroplasts and other organelles in leaves with various rates of photosynthesis.Plant Physiol.47: 15–23.
Botha, C.E.J. 1992. Plasmodesmatal distribution, structure and frequency in relation to assimilation in C-3 and C-4 grasses in southern Africa.Planta187: 348–358.
Brown, W.V. 1975. Variations in anatomy, associations, and origins of Kranz tissue.Amer. J. Bot.62: 395–402.
Brown, R.H., J.H. Bouton, L. Rigsby and M. Rigler. 1983. Photosynthesis of grass species differing in carbon dioxide fixation pathways.Plant Physiol.71: 425- 431.
Carolin, R.C., S.W.L. Jacobs and M. Vesk. 1973. The structure of the cells of the mesophyll and parenchymatous bundle sheath of the Poaceae.Bot. J. Linn. Soc.66: 259–275.
Dengler, N.C., R.E. Dengler and P.W. Hattersley. 1985. Differing ontogenetic origins of PCR (“Kranz”) sheaths in leaf blades of C-4 grasses (Poaceae).Amer. J. Bot.72: 284–302.
Dengler, N.C., R.E. Dengler and P.W. Hattersley. 1986. Comparative bundle sheath and mesophyll differentiation in the leaves of the C-4 grassesPanicum effusum andP. bulbosum.Amer. J. Bot.73: 1431–1442.
Dengler, N.C., R.E. Dengler, P.M. Donnelly and M.F. Filosa. 1995. Expression of the C4 pattern of photosynthetic enzyme accumulation during leaf development inAtriplex rosea (Chenopodiaccae).Amer. J. Bot.82: 318–327.
Dengler, N.C., P.M. Donnelly and R.E. Dengler. 1996. Differentiation of bundle sheath, mesophyll, and distinctive cells in the C4 grassArundinella hirta (Poaceae).Amer. J. Bot.83: 1391–1405.
Giglioli-Guivarch, N., J.N. Pierre, S. Brown, R. Choilet, J. Vidal and P. Gadal. 1996. The light-dependent transduction pathway controlling the regulatory phosphorylation of C-4 phosphoenolpyruvate carboxy- läse in protoplasts fromDigitaria sanguinulis.Plant Cell8: 573–586.
Gutierrez, M., V.E. Gracen and G.E. Edwards. 1974. Biochemical and cytological relationships in C-4 plants.Planta119: 279–300.
Hatch, M.D. 1987. C-4 photosynthesis: a unique blend of modified biochemistry. anatomy and ultrastructure.Bioch. et Biophys. Acta895: 81–106.
Hatch, M.D., T. Kagawa and S. Craig. 1975 Sub-division of C-4-pathway species based on differing C- 4 acid decarboxylating systems and ultrastructural features.Aust. J. Pl. Physiol.2: 111–128.
Hattersley, P.W. 1984. Characterization of C-4 type leaf anatomy in grasses (Poaceae). Mesophyll: bundle sheath area ratios.Ann. Bot.53: 163–179.
Hattersley, P.W. and A.J. Browning. 1981. Occurrence of the suberized lamella in leaves of grasses of different photosynthetic types. I. In parenchymatous bundle sheaths and PCR (“Kranz”) sheaths.Protoplasma109: 371–401.
Kolattukudy, P.E. 1980. Biopolyester membranes of plants: cutin and suhcrin.Science208: 990–1000.
Laetsch, W.M. 1974. The C-4 syndrome: A structural analysis.Ann. Rev. Plant Phvsiol.25: 27–52.
Langdale, J.A., M.C. Metzler and T. Nelson. 1987 The argentia mutation delays normal development of pholosynthetic cell-types in Zea mays.Dev. Biol.122: 243–255.
Nelson, T. and J.A. Langdale. 1989. Patterns of leaf development in C-4 plants.Plant Cell1: 3–13.
Robinson-Beers, K. and R.F. Evert. 1991. Fine structure of plasmodesmata in mature leaves of sugarcane.Planta184: 307–318.
Sinha, N.R. and E.A. Kellogg. 1996. Parallelism and diversity in multiple origins of C4 photosynthesis in the grass family.Amer. J. Bot.83: 1458–1470.
Spurr, A.R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy.J. Ultrastruct. Res.26: 31–43.
Taylor, C.B. 1996. C3 or C4? Maize mutations and elaboration of Kranz anatomy.Plant Cell8: 761–762.
Voznesenskaya, E.V. and Y.V. Gamalei. 1986. Ultrastructural characteristics of leaves with Kranz anatomy.Bot. Zhur.71: 1291–1306.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, I., Pak, JH., Seo, B. et al. Ultrastructural aspects of kranz anatomy inDigitaria sanguinalis andSetaria viridis (poaceae). J. Plant Biol. 40, 102–109 (1997). https://doi.org/10.1007/BF03030241
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03030241