Abstract
In taxonomic studies, in addition to floral characteristics, the structural characteristics of the vegetative organs also contribute to the taxonomic determination of the species. To provide information regarding these characteristics in the genus Tetramerium, a structural, micromorphological and histochemical analysis of cross sections of the leaves was performed using histochemical techniques and optical and scanning electron microscopy of two of its species, T. glutinosum, endemic to Mexico, and the widely distributed T. tenuissimum, was conducted. The two species presented amphistomatic leaves; double palisade chlorenchyma on adaxial and abaxial surfaces, leaf unifacial; vascular bundle sheaths with kranz anatomy; intradermal and subepidermal cystoliths of various shapes and sizes; nonglandular trichomes osteolate with a thin-walled conical head, glandular trichomes, including a new type, the straight, bright-ringed tricellular trichomes, and a variety of multicellular glandular trichomes. The glandular trichomes secrete waxes or oleoresins and mucilage deposited on the surfaces abaxial and adaxial in the form of platelets, granules and threads or strands. The histochemistry of the cystoliths highlights the presence of proteins and polysaccharides as a product of the possible superposition of the cell wall and plasmalemma lamellae. All these characteristics are typical of species from semi-arid habitats and correspond to the defense function against biotic and abiotic agents assigned to trichomes and their secretions in other studies of various genera and families, as well as to the adaptation function to these habitats of the kranz structure, which was novel for the genus Tetramerium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The family Acanthaceae Juss., which is mainly pantropical, comprises approximately 4320 species of 240 genera arranged in four subfamilies: Nelsonioideae Pfeiffer, Acanthoideae Eaton, Thunbergioideae T. Anderson and Avicennioideae Miers. Acanthoideae is the largest subfamily and is a monophyletic taxon and Tetramerium CF Gaertn. belongs to the subfamily Acanthoideae and the tribe Justiceae Dumortier (Daniel 1986; Stevens 2001; Kiel et al. 2018).
Acanthaceae is one of the families listed by Metcalfe and Chalk (1965) that presents cystoliths, typically on the leaf (Larcher and Boeger 2006). The genus has nearly 200 species, where 75% come from the New World (MacDade et al. 2018). From the list of Tetramerium species that appear in tropicos.org, 30 species remain when the synonyms are eliminated and correspond to the number of species for the genus, which appear in POWO (powo.science.kew.org/taxon/327080). It is distributed from the southwestern USA to central South America, and the distribution correlates with the semiarid tropical deciduous forest that extends almost continuously from Mexico to northern South America (Daniel 1986; Stevens 2001).
Tetramerium species are recognized for presenting conspicuously tetrangular spikes, one pair of bracteoles that accompany each flower, two stamens with dithecal anthers and without basal appendages, tricolporate pollen with six pseudocolpi, stipitate capsules and four or fewer glabrous seeds. Cystoliths are present on the vegetative surfaces of their stems, petioles, leaves and bracts; they are elongated, straight to slightly curved and rounded or conical at the tips, sometimes almost globose and with lengths of 0.05 to 0.30 mm. (Daniel 1986). Tetramerium glutinosum Lindau is a fragrant shrub up to 1 m tall. It has petiolate leaves with pubescent surfaces and margins and straight, glandular and nonglandular trichomes 0.05 to 0.10 mm long. Tetramerium tenuissimum Rose is a perennial, herbaceous, erect or spreading shrub up to 80 cm in height. It has petiolate leaves with nonglandular trichomes on both surfaces (Stevens 2001; Daniel and Acosta-Castellanos 2003). Field observations revealed that both species under study remain alive during the winter and flower during this period with inflorescences protected by photosynthetic bracts.
Both species did not present a report of ethnobotanical use in the herbarium specimens reviewed, as is the case of Justicia spicigera (muicle, popular name in Spanish and indigenous language) (Zolla and Argueta 2009), a medicinal plant belonging to the same Justiceae tribe.
In addition to the morphological characteristics of reproductive organs, structural characteristics of vegetative organs such as stems, petioles and leaves are now also used in the classification of species (Al-Hakimi et al. 2022; Raza et al. 2022).
In the Acanthaceae family, several reports describe morphological and structural characteristics of leaves such as the epidermis and mesophyll, the types of stomata, the presence of glandular and nonglandular trichomes, the shape and distribution of the cystoliths and the shape of the vascular bundles (Sreemadhavan et al. 1968; Inamdar et al. 1990; Larcher and Boeger 2006; Bhatt et al. 2010; Patil and Patil 2011; Choopan and Grote 2015; Fisher et al. 2015; Fernandes and Krishnan 2019; Gabel et al. 2021; Steyn and Van Wyk 2021).
Following this trend, this study analyzed the micromorphology of the epidermis and the structure of the mesophyll of the leaves of two species of the Tetramerium genus, one endemic to the Balsas River Region of Mexico, T. glutinosum, and the other widely distributed in Mexico, T. tenuissimum, in environments with rainfall in summer and a long period of drought, from October to May, that exposes them to a water deficit, with average maximum temperature of 35 °C and minimum of 21 °C annually. The objective was to investigate the micromorphological and anatomical adaptations of the leaves to the semiarid habitats of the species, looking for structures similar to other Acanthaceae species, which develop in the same habitats.
2 Materials and methods
Plant material
– The leaves of the species under study were collected by the Master of Science Rosa María Fonseca-Juárez, in Cerro Tuxpan, N 18° 22′ 618″ and W 99° 28′ 149″ to 1,050 msnm and in El Naranjo inside the Cañón de la Mano, Iguala, Gro., at N 18° 23′ 59.35″ and W 99° 31′ 59.12″ to 820 msnm, Guerrero, Mexico. The herbarium specimens were deposited in the Herbarium of the Faculty of Sciences (FCME) of the National Autonomous University of Mexico under the following reference vouchers: T. glutinosum # 53,290 and T. tenuissimum #53,289. Mature leaves from branch with inflorescence were selected from four plants of each species collected in the field, and the leaves were used divided in the apical, median and basal third.
Leaf anatomy and histochemistry
– The anatomical and histochemical study was performed using the Johansen method (Johansen 1940). The samples were fixed in a mixture of formaldehyde, ethanol and acetic acid (FAA, 10%:50%:5% + 35% water) (Merck, S.A. de C.V., Naucalpan de Juárez, México) (Johansen 1940) for 5 d, washed with tap water for 4 h and then subjected to a dehydration process in ascending concentrations of ethanol (30%, 50%, 70%, 85%, 96% and 100%) with two changes over 20 min per dehydrating solution. For the preinclusion stage, the samples were immersed in ethanol–xylene mixtures (1:1 and 1:2) for 10 min each, xylene for 20 min and xylene–paraffin mixtures (1:1 and 1:2) for 30 min each. The samples were then embedded in paraffin at 54 °C, and the samples remained in pure paraffin for 12 h before being placed in blocks for sectioning. Sections were made with a rotary microtome (MICROM HM 340E) at a thickness of 10 μm and placed on sequentially numbered slides for later comparison of similar areas with different stains (Sigma-Aldrich Co., Milwaukee, WI, EE. UU.): naphthol black blue (NBB) to detect proteins, periodic acid-Schiff’s (PAS) reagent to detect insoluble polysaccharides and Johansen’s quadruple (JQ) stain to detect various molecules (Johansen 1940). No controls were used, the results were only interpreted based on what was described by Johansen (1940) and on our own previous works (Laguna-Hernández et al. 2017; Brechú-Franco et al. 2021). Observations were made using bright-field and phase-contrast microscopy (Olympus BX41) and were photographed with a digital camera (Evolution MP Color Media Cybernetics).
Scanning electron microscopy
– For micromorphological analysis under scanning electron microscopy (Bozzola and Russell 1999), the samples were dehydrated in a graded ethanol series and critical point dried using a CPD 030 critical point dryer (Bal-Tec AG, Liechtenstein). The samples were mounted on aluminum stubs using carbon double-sided tape and coated with gold by means of a Denton Vacuum Desk II (Denton Vacuum, Moorestown, N.J., USA). Observations were carried out with Jeol JSM 5310-LV scanning electron microscopes (JEOL, Ltd., Tokyo, Japan). The description of the trichomes was based on the report by Payne (1978).
3 Results
Tetramerium glutinosum Epidermis
– The leaf presented rounded edges, an epidermis with sinuous cells averaging 30 µm long by 18 µm wide and 16 µm high; a thick cuticle was evident with JQ staining and scanning electron microscopy. Diacytic stomata were present on both the abaxial and adaxial surfaces (an amphistomatic leaf) (Fig. 1a–c, Fig. 2a, j, k). Trichomes were present on both abaxial and adaxial surfaces and the majority were glandular trichomes of the following types: (a) globular peltate trichome: trichomes of various sizes with a tricellular stalk from 100 to 150 μm long (others longer, from 170 to 240 μm) with the thickened annular base protruding from the epidermis (the intercellular junctions also thickened) and a multicellular peltate globose head with up to eight cells 30 to 45 µm in diameter, which were more abundant on the abaxial side of the leaf (Fig. 1a, d, Fig. 2a–d; (b) brevicollate trichomes: with a small pyramidal stalk cell of 13 μm and a globose unicellular or bicellular head of 30 μm in diameter, which expels secretions through a circular lateral groove created by the rupture of the wall and the cuticle (when only one cell) or through lateral pores (when they are two cells) and ultimately takes the form of a wrinkled disk abundant on the adaxial side (Fig. 1e, k, Fig. 2d, f, k); (c) clavate trichome: bicellular stalked trichomes 40 μm long with a globose pyramidal four-celled clavate head 16 μm in diameter, which secrete through lateral pores in the cells (Fig. 1g, 2e); (d) globular head trichome: bicellular stalked trichomes 40 µm long with a peltate globose head of one or two cells 16 µm in diameter (Fig. 1j, Fig. 2c, j–l); (e) straight, bright-ringed, tricellular, acuminate trichome: with an intermediate cell that appears as a bright ring in optical microscopy (hence the description of straight, bright-ringed, tricellular, acuminate trichomes) approximately 40 μm long and a terminal cell (under SEM) that takes the form of a thin-walled hood, broken and with the presence of granular and thread-like secretory material that is also observed in the form of clumps in the basal cell (Fig. 1b, f, g, j–l, Fig. 2b–e, g–l); (f) long tricellular attenuate trichomes ending in a thin wall (in some cases broken with clumps of secretion adhered), the intercellular junctions thickened in an annular shape similar to osteolate, 200 to 300 μm long with secretion material observed inside the cells in optical microscopy (Fig. 1h, i, Fig. 2a–d, g, h); and (g) short attenuate trichome: unicellular attenuate trichomes of approximately 60 μm that are smooth-walled with a thickened base and a thin-walled tip, sometimes broken (Fig. 1b, j, k, Fig. 2e–g). On the epidermis between the trichomes, SEM revealed clumps of secretory granules and platelets that could be oleoresins or waxes and mucilages (Fig. 2g, h, l, n) (Table 1).
Tetramerium glutinosum leaf. Light microscopy micrographs. a, b, d–l Cross section; c Paradermal section; a-c Rounded edge, epidermis, sinuous cells, partially covered by the glandular trichomes, diacytic stomata, substomatic chamber, intradermal cystoliths and between the chlorophyll palisade parenchyma; d–i Different types of glandular trichomes: attenuate trichome; brevicollate trichome; straight, bright-ringed, tricellular, acuminate trichome; clavate trichome; globular head trichome; globular peltate trichome; j, k Double palisade parenchyma on both sides and broad U-shaped collateral vascular bundle of the midrib, bundle sheath cells around the vascular bundles, l intradermal cystolith projected to the chlorophyll parenchyma; a, c–i, l JQ staining; b NBB reaction; j, k NBB-PAS; e, g, i Phase contrast microscopy. att, attenuate trichome; bct, brevicollate trichome; brt, straight, bright-ringed, acuminate trichome; bs, bundle sheath cells; clt, clavate trichome; *, cystolith; ght, globular head trichome; gpt, globular peltate trichome; pp, palisade parenchyma; sc, sinuous cells; ssc, substomatic chamber; st, stomata; vb, vascular bundle. JQ Johansen’s quadruple staining, NBB Naphthol Blue Black reagent, PAS periodic acid-Schiff reagent
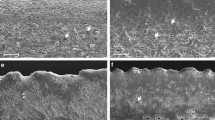
Tetramerium glutinosum leaf. Scanning electron microscopy (SEM) micrographs. a–l Micromorphological properties of leaf surfaces with different types of glandular trichomes: attenuate trichome; brevicollate trichome; straight, bright-ringed, tricellular, acuminate trichome; clavate trichome; globular head trichome; globular peltate trichome; g, h, l, n Secretory granules and platelets of oleoresins or waxes and mucilages; m Double palisade parenchyma on both sides, bundle sheath cells around the vascular bundles; o intradermal cystolith projected to the chlorophyll parenchyma. att, attenuate trichome; bct, brevicollate trichome; brt, straight, bright-ringed, acuminate trichome; bs, bundle sheath cells; clt, clavate trichome; *, cystolith; ght, globular head trichome; gpt, globular peltate trichome; pp, palisade parenchyma; sc, sinuous cells; ssc, substomatic chamber; st, stomata; vb, vascular bundle; w, wax
Mesophyll
– With optical microscopy and scanning electron microscopy, a double palisade chlorophyllous parenchyma was observed on both sides of the leaf (a unifacial leaf) with long cells averaging 30 µm and wide intercellular spaces corresponding to the substomatic chambers (Fig. 1b, j, Fig. 2m). The rib or central vein presented a wide U-shaped collateral vascular bundle that also presented in the secondary veins. Along the vascular bundles and surrounding them, a row of large isodiametric cells was observed with chloroplasts, similar to the sheath of the kranz anatomy (Fig. 1b, f, k, Fig. 2m). Between the epidermal cells on both sides, abaxial and adaxial, and between the parenchymal cells of the vascular bundle were large globose cystoliths that appeared circular in cross section along with some irregular shapes with protrusions characteristic of cystoliths; all projected toward the chlorophyllous parenchyma and to the outside, giving the epidermis a rough appearance. The size of the cystoliths ranged from 93 µm in length by 30 to 48 µm in width or diameter. The cystoliths showed a positive reaction to the NBB reagent for proteins and to the PAS reaction for insoluble polysaccharides (Fig. 1a, b, j–l, Fig. 2o) (Table 2).
Tetramerium tenuissimum—Epidermis
– In the sections, the leaf presented rounded edges, and the epidermis had sinuous cells averaging 28 µm long by 10 µm wide and 10 µm high and a thick cuticle with a rough appearance was evident with JQ staining and with SEM (Fig. 3a, 4p). Diacytic stomata were present on both abaxial and adaxial surfaces (an amphistomatic leaf) (Fig. 3a, b, d, Fig. 4b, c) and densely covered by trichomes. Glandular trichomes were of the following types: (a) brevicollate or surculate trichomes with a small pyramidal stalk cell of approximately 12 μm and a globose head of one or two cells 20 μm in diameter that expel secretions through a circular lateral groove created by the rupture of the wall and the cuticle (when it is only one cell) or through lateral pores (when there are two cells) and ultimately take the form of a wrinkled disc, which are abundant on the adaxial side (Fig. 3i, j, Fig. 4d, g, i–k); (b) straight, bright-ringed, tricellular, acuminate trichomes with a robust base of approximately 26 μm in diameter comprising four cells and protruding from the epidermis with the basal wall and stalked cells adorned with spicules, probably of wax and a hood-shaped terminal cell that is mostly broken or burst with the secretion product only observed with SEM attached to and around the cell and the epidermis and measuring approximately 40 µm long (Fig. 3a–c, e, Fig. 4 l–p); (c) clavate trichome, tricellular stalked trichomes between 50 and 70 μm with the third smallest cell attached to a subglobose four-celled clavate head approximately 18 μm long with a robust four-celled pedestal-shaped base and secretions occurring through lateral ruptures (Fig. 3k, Fig. 4a, f–h. p); (d) unicellular attenuate trichomes, 35 μm to 70 μm with a robust base of 4 cells protruding from the epidermis and a thin tip, sometimes with secretions, and the cell wall adorned with spicules, probably of wax, abundant on the abaxial side (Fig. 3f, Fig. 4g, i, k); and (e) attenuate trichomes of two to four cells, clearly cylindrical in shape, with the first cell generally approximately 30 μm and the others of different lengths (the second typically 50 μm and the last up to 130 μm, which is the longest), with a thin-walled tip, broken due to the expulsion of waxes, these trichomes are distinguished by thickened intercellular junctions, also with spicules (Fig. 3d, Fig. 4e, j). Nonglandular trichomes of variable size were observed, but the majority were approximately 3 mm, tricellular, osteolate with a prominent base and intercellular junctions thickened in an annular form and cell walls adorned with spicules, generally distributed at the edges (Fig. 4a). With SEM, an abundant deposit of waxes in the form of platelets and granules, forming thick lumps, was observed on the abaxial and adaxial surfaces. In some areas, threads or strands were observed coming out of the clumps, and many came out of the hood-headed trichomes and were present on them (Fig. 4c, l–o) (Table 1).
Tetramerium tenuissimum leaf. Light microscopy micrographs. a–k Cross section; a–c Rounded edge, epidermis, partially covered by the glandular trichomes, diacytic stomata, substomatic chambers, intradermal cystoliths and between the chlorophyll palisade parenchyma, double palisade parenchyma on both sides, bundle sheath cells with chloroplasts, broad U-shaped collateral vascular bundle of the midrib; a–c, g, i, j bundle sheath cells around the vascular bundles with chloroplasts; d-k different types of glandular trichomes: attenuate trichome; brevicollate trichome; straight, bright-ringed, tricellular, acuminate trichome; clavate trichome; a–k JQ staining, e, h, j, k Phase contrast microscopy. att, attenuate trichome; bct, brevicollate trichome; brt, straight, bright-ringed, acuminate trichome; bs, bundle sheath cells; clt, clavate trichome; *, cystolith; pp, palisade parenchyma; sc, sinuous cells; ssc, substomatic chamber; st, stomata; vb, vascular bundle. JQ Johansen’s quadruple staining
Tetramerium tenuissimum leaf. Scanning electron microscopy (SEM) micrographs. a–l Micromorphological properties of leaf surfaces with diacytic stomata, the nonglandular trichome: osteolate, and different types of glandular trichomes: attenuate trichome; brevicollate trichome; straight, bright-ringed, tricellular, acuminate trichome; clavate trichome; c, l–o Deposit of waxes in the form of platelets and granules, many came out of the hood-headed trichomes; p–r Double palisade parenchyma on both sides, bundle sheath cells around the vascular bundles; q, s, t intradermal cystoliths projected to the chlorophyll parenchyma. att, attenuate trichome; bct, brevicollate trichome; brt, straight, bright-ringed, acuminate trichome; bs, bundle sheath cells; clt, clavate trichome; *, cystolith; pp, palisade parenchyma; sc, sinuous cells; ssc, substomatal chamber; st, stomata; vb, vascular bundle; w, wax
Mesophyll
– The mesophyll presented a palisade chlorophyllous parenchyma with two layers of elongated cells, approximately 20 to 30 µm long, loosely arranged with intercellular spaces corresponding to the substomatic chambers on both sides of the leaf. In the central vein and in the secondary veins, there was a wide U-shaped collateral vascular bundle surrounded by large isodiametric cells, which pass between the palisade parenchyma and give the appearance of a kranz anatomy. Using SEM, the arrangement and typical shape of the sheath cells were observed (Fig. 3a–d, g–k, Fig. 4p–r). Between the epidermal cells on both sides, cystoliths of circular and irregular appearance were observed with a diameter between 30 and 40 µm, which projected toward the palisade parenchyma and to the outside, giving the epidermis a rough appearance. In some lithocysts, the deposition of a refractive granular material was observed under polarized light, but the chemical composition was not determined (Fig. 3a–d, Fig. 4q, s, t) (Table 2).
4 Discussion
Anatomical characteristics, in addition to morphological ones, are fundamental to differentiating plant species in their vegetative state or as a complement to floral characteristics (Nurul-Aini et al. 2014; Gul et al. 2018; Gul et al. 2019; Karaismailoğlu and Güner 2021; Zhao et al. 2022). In this study, using histochemical techniques and optical and scanning electron microscopy, distinctive characteristics were observed on the surfaces and in the cross sections of the leaves such as the distribution and shape of the cystoliths, the structural variation and the shapes of the vascular bundles and epidermal characteristics of the leaves such as the type of stomata and the types of trichomes, which could have taxonomic significance for the genus and for the family Acanthaceae.
Epidermis
– Observations with scanning electron microscopy showed the epidermis to have a protective barrier composed of thick cuticles on which waxes, abundant in T. tenuissimum, are deposited. Histochemistry with JQ revealed its highly hydrophobic lipidic nature, which prevents the loss of water and acts as a defense against biotic or abiotic agents, as seen in the genus Satanocrater Schweinf. (Acanthaceae), where the presence of waxes has been proposed as part of the xeric adaptations in this genus endemic to arid tropical Africa (Tripp and Fatimah 2012). This role is due to two basic components common to all cuticles: cutin and waxes, which contain polyesters composed of C16 and C18 hydroxy fatty acids and glycerol and a mixture of C24 to C34 alkanes, alcohols, ketones and wax esters (Barthlott et al 1998; Nawrath 2002; Kunst and Samuels 2003; Javelle et al. 2011). Both species in this study were amphistomatic with wide intercellular spaces in the palisade parenchyma, which correspond to the substomatic chambers that connect with diacytic stomata. The diacytic stomata is the most common type of stoma in Acanthaceae (Larcher and Boeger 2006; Patil and Patil 2011; Abdel-Hameed 2015; Al-Hakimi et al. 2022; Raza et al. 2022). The amphistomatic and unifacial leaves observed in the studied species are characteristic of plants where light falls on both sides of the leaf and of plants that inhabit xeric environments (Esau 1977; Dörken et al. 2023). Another adaptation to these environments is an epidermis that secretes mucilaginous substances, which protect the stomata from environmental desiccation (Esau 1977; Naidoo et al. 2021). We assume that the secretion products in the Tetramerium species in this study are oleoresins or a mixture of waxes and mucilages due to the sticky nature of the leaves to the touch, the fragrance of plants and the solubility of them in xylene used in the histochemical technique (Burdock 2010). Processing for scanning electron microscopy did not dissolve the epicuticular waxes, which is why the natural organization of clumps, platelets and threads was maintained, which are similar to those described by Barthlott et al. (1998), Shepherd and Whynne Griffiths (2006), Koch and Ensikat (2008).
Trichomes
– The two Tetramerium species showed a dense layer of glandular trichomes on both leaf surfaces, although the taxonomic descriptions of these species mention straight glandular and nonglandular trichomes for T. glutinosum and only nonglandular trichomes for T. tenuissimum (Daniel 1986; Daniel and Acosta-Castellanos 2003). The dense layer of trichomes, according to Esau (1977), can increase the reflective surface area, protecting against environmental conditions with high irradiance and limiting air movement, which minimizes water loss in the dry environment, thereby reducing leaf temperature. In this study, trichomes of various types were found in these species, some of which have been described for other species of Acanthaceae and for other families like Lamiaceae (Ascensão and Pais 1998; Naidoo et al. 2021). However, straight, bright-ringed, tricellular, acuminate trichomes were found in both species, which can be considered a distinctive character within the genus because there are no reports for other species of Acanthaceae. They are also differentiated by the presence of long bi- or tricellular attenuate trichomes with a sharp tip, particularly distributed along the leaf edges, and their walls are covered with spicules in T. tenuissimum, which Naidoo et al. (2021) described as micropapillae in the species Leucas lavandulaefolia Rees (Lamiaceae) and may be similar to those described for species of Mendoncia Bremek., Ruttya Ruttya and Lepidagathis Willd. (Acanthaceae) (Ahmad 1978). We propose that the spicules are lipidic in nature, derived from the secretion of trichomes, because they were also lost during the process of histochemical techniques, like the waxes of the epidermis. The unicellular attenuate trichomes with a smooth wall, thickened base and a slender tip are distinguished as characteristic of the species T. glutinosum; we characterized these as glandular because scanning electron microscopy revealed the presence of secretion material in the basal cell and sometimes in the broken tip of the trichome. Trichomes similar to these have been reported for species of the genus Solanum and described as attenuate glandular with a small glandular tip or as attenuate basillatus glandular hair (Watts and Kariyat 2021) and characterized as nonglandular in the species Lepidagathis trinervis Nees (Acanthaceae) (Ahmad 1978) and are possibly equivalent to those found in Acanthus ebracteatus Vahl (Acanthaceae) (Nurul-Aini et al. 2014). Glandular trichomes with a clavate head and a pedestal constituted by four large cells were observed to be rare in T. glutinosum and more abundant in T. tenuissimum and could correspond to those reported for Hygrophila serpyllum T. Anderson (Acanthaceae) (Ahmad 1978), but no other report was located for Acanthaceae, although they are similar to those located in species of Arnica Boehm. (Asteraceae) (Muravnik et al. 2022) and Solanum Sendtn. (Solanaceae) (Watts and Kariyat 2021). T. tenuissimum was characterized by tricellular stalked glandular attenuate trichomes with a thickened base and annular intercellular junction and a terminal cell in the shape of a curved or sinuous tip with a thin wall. The short-stalked trichomes of one pyramidal cell with a globose head of one or two cells, brevicollate, are similar to those described in some genera of Lamiaceae: Leucas R. Br., Pogostemon Briq., Plectranthus L'Hér in which they are called capitate and peltate trichomes (Ascensão and Pais 1998; Guo and Zhou 2018; Dias-Machado et al. 2021; Naidoo et al. 2021). They are also similar to those described for some species of Acanthaceae with a panduriform head (Ahmad 1978) and to those reported for Acanthus ilicifolius L. (Nurul-Aini et al. 2014) and some species of Acanthopsis Harv. (Steyn and Van Wyk 2021). Thus, this type of trichome is common in Acanthaceae and in other families, and they are not taxonomic determinants for these species. In the species studied, the abundance of thick-walled trichomes can function as protective reflectors that can reduce photoinhibition, decrease damage to leaf photochemistry by UV-B (Bickford 2016), favor optimal temperatures for photosynthesis in hot and arid environments (Ehleringer and Mooney 1978), promote photosynthesis by absorbing wavelengths in the far infrared range (Takeda et al. 2013) and efficiently use water (Baldocchi et al. 1983; Bhatt et al. 2010). Glandular trichomes produce a wide variety of compounds that provide direct or indirect protection against herbivores and pathogens (Kim et al. 2012). The two species studied in this work presented glandular trichomes that could provide this type of protection to plants, but only T. glutinosum is reported as a fragrant shrub (Daniel 1986). However, the secretion of waxes or oleoresins as granules and platelets may also be related to this protective function and to the habitat of the species, as discussed in the work by Barthlott (1998). The tricellular stalked with four-celled globose-headed trichomes and brevicollate trichomes of Tetramerium species is similar to the capitate trichomes of L. lavandulaefolia and Pogostemon auricularius (L.) Hassk., which contain small amounts of essential oils, phenolic compounds and mucilage in their small subcuticular spaces. It is speculated that these viscous secretions possess antiherbivory activity such as foraging deterrents or insect repellents (Naidoo et al. 2021). We observed that the form of the secretions of capitate peltate globose and clavate trichomes may be similar to that described in Pogostemon auricularius (Guo and Zhou 2018) and Ocimum obovatum E. Mey. ex Benth. (Naidoo et al. 2013) (Lamiaceae) because they presented a unique wrinkled structure with pores or grooves.
– The thread-like secretions could be the result of partial solubility in ethanol when processing for observation under scanning electron microscopy. In the samples processed for histochemistry, only the fibrous and granular content of the secretion was observed within the trichomes, but not on the surface, which may indicate that the surface secretions were dissolved in the process used for fixation and preinclusion, but the secretion product in the interior of the trichome cells was not affected. Similarly, due to the presence of spicules on the walls of the trichomes in T. tenuissimum, we propose that they are lipidic in nature (waxes) because they were observed with scanning microscopy but not in histochemical preparations. The presence of glandular trichomes and secretion waxes in these Tetramerium species is important for their taxonomic status because in the taxonomic description of the species T. tenuissimum, only the presence of nonglandular trichomes is mentioned (Stevens 2001; Daniel and Acosta-Castellanos 2003) and for neither species is the presence of waxes or sticky substances mentioned.
Cystoliths
– The cystoliths in these species follow the shape and size patterns described in foliar anatomy studies of Acanthaceae species, which can be elongated with protruding edges and rounded or pointed ends (Inamdar et al. 1990; Larcher and Boeger 2006; Patil and Patil 2011; Choopan and Grote 2015; Lande 2017; Gabel et al. 2021). Particularly useful for differentiation are those that accompany the vascular bundles. Furthermore, in the case of T. glutinosum and T. tenuissimum, the characteristic of being intradermal and projecting to the chlorophyll parenchyma and to the outside makes them unique, giving the surfaces a rough texture or forming a foliar epidermal impression (Koch et al. 2009); this structure is consistent with the characteristics of its habitat: rocky slopes, along streams and in disturbed areas. In general, cystoliths are said to function in fixing and mobilizing carbonate salts and therefore participate in the water regulation of the plant (Ajello 1941). Gal et al. (2012) provided support for a role in light scattering. Kai and Okazaki (2003) proposed a role as cellular pH stats during nitrite assimilation. Okamoto and Rodella (2006) found that silkworms preferred feeding on mulberry leaves (Moraceae) from cultivars with a lower density of cystoliths, supporting an antiherbivory function. Based on the histochemical results in this study, the presence of proteins and polysaccharides in their structure is notable and is consistent with the studies of their origin, in which it was established that they are the product of cell wall overlap (Nitta et al. 2006; Gal et al. 2012; Gabel et al. 2021); we add the observation that the plasma membrane of the cell accompanies the cell wall, also in the form of lamellae. Therefore, the cystolith can be a structure that not only provides a large organic surface that protects the leaf from solar radiation and favors photosynthesis (Setoguchi et al. 1989; Gal et al. 2012; Pierantoni et al. 2017, 2018) but also, due to its protein nature and highly hydrophilic polysaccharides, could be closely involved in water regulation and the mobilization system of carbonated salts (Ajello 1941).
Mesophyll
– The two Tetramerium species presented a wide U-shaped collateral vascular bundle of the midrib, similar to the Strobilanthes Blume species S. heyneana Nees, S. barbata Nees and S. lupulina Nees reported by Fernandes and Krishnan (2019). The two species of Tetramerium have a sheath with large isodiametric cells typical of the kranz anatomy, which in the genus Blepharis Juss. is proposed as the culmination of anatomical and biochemical progression through an intermediate C3–C4 phase (Sage et al. 2013; Fisher et al. 2015). Of the species of Blepharis described by Fisher et al. (2015), Blepharis grossa T. Anderson, B. calcitrapa Benoist and B. katangensis De Wild. have a mesophyll structure similar to that of the Tetramerium species, particularly due to the presence of a double palisade chlorenchyma on both sides of the leaf in both species, which is identical to the genus Flaveria Juss., as seen in F. ramosissima Klatt and F. floridana J.R. Johnst. (Asteraceae) (McKown and Dengler 2007). In the report for Ruelliopsis setosa C.B. Clarke (Acanthaceae), a layer of large hyaline cells is referred to between the two palisade parenchyma, but they are not referred to as bundle sheath cells (Tripp and Fekadu 2014), although they are similar to the bundle sheath cells of the Tetramerium species under study. In addition, the characteristics of unifacial leaves could also indicate adaptation to an arid environment, allowing more efficient and accelerated gas exchange and water transport, which increases photosynthesis (Burrows 2001).
– In accordance with the above, the Tetramerium species studied here could present this type of intermediate C3–C4 or C4 photosynthesis, which is consistent with the warm and dry habitat of the two species, particularly that of T. glutinosum as an endemic to the Balsas River Region of Mexico (Daniel et al. 2008), and similar to species intermediate to types C3 and C4 (C3–C4) such as Panicum L., Neurachne S.T. Blake, Flaveria Juss.and Moricandia DC. (Brown and Hattersley 1989).
– The findings about the structure of the mesophyll of the leaves of these species, similar to the kranz anatomy, the unifacial characteristic, the micromorphological characteristics of the epidermis, such as the presence of waxy and mucilaginous substances, the abundance and variety of trichomes and the characteristics of the cystoliths, correspond to adaptive aspects of the species to semi-arid habitats, consistent with species of other genera, available for the taxonomy of the Acanthaceae family.
The findings of this study make it necessary to continue a line of research on the genus Tetramerium of Mexico and the American continents, particularly of the arid zones and adjacent regions, to determine which species share the kranz anatomy and the other adaptive traits available for the genus, and the paired and higher clades.
References
Abdel-Hameed UK (2015) Phenetic analysis of morphological and molecular traits in Acanthaceae Juss. J Biosci Med 3:18–34
Ahmad KJ (1978) Epidermal hairs of Acanthaceae. Blumea 24:101–117
Ajello L (1941) Cytology and cellular interrelations of cystolith formation in Ficus elastica. Am J Bot 28:589–594
Al-Hakimi AS, Razak SA, Saeed AA, Latiff A (2022) Anatomical study of selected species of tribe Acantheae (Acanthoideae, Acanthaceae) from Yemen. Malay Nat J 74:31–46
Ascensão L, Pais MS (1998) The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Ann Bot 81:263–271
Baldocchi DD, Verma SB, Rosenberg NJ, Blad BL, Garay A, Specht JE (1983) Leaf pubescence effects on the mass and energy exchange between soybean canopies and the atmosphere. Agron J 75:537–543
Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126:237–260
Bhatt A, Naidoo Y, Nicholas A (2010) The foliar trichomes of Hypoestes aristata (Vahl) Sol. ex Roem. & Schult var aristata (Acanthaceae) a widespread medicinal plant species in tropical sub-Saharan Africa: with comments on its possible phylogenetic significance. Biol Res 43:403–409
Bickford CP (2016) Ecophysiology of leaf trichomes. Funct Plant Biol 43:807–814
Bozzola JJ, Russell LD (1999) Electron microscopy: principles and techniques for biologists, 2a edn. Jones & Bartlett Publishers, Boston USA, p 670
Brechú-Franco AE, Larqué-Saavedra AF, Laguna-Hernández G, Pasillas-Rodríguez K, Espinosa-Matias S (2021) Morphology, structure, and histochemistry of the inflorescences, fruit, and seed of the Ramón nut, Brosimum alicastrum Sw. subsp alicastrum CC Berg (Moraceae). Braz J Bot 44(2):457–466. https://doi.org/10.1007/s40415-021-00708-w
Brown RH, Hattersley PW (1989) Leaf anatomy of C3–C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91:1543–1550
Burdock GA (2010) Fenaroli’s handbook of flavor ingredients, 6th edn. Taylor & Francis
Burrows GE (2001) Comparative anatomy of the photosynthetic organs of 39 xeromorphic species from subhumid New South Wales, Australia. Int J Plant Sci 162:411–430
Choopan T, Grote PJ (2015) Cystoliths in the leaves of the genus Pseuderanthemum (Acanthaceae) in Thailand. NU Int J Sci 12:13–20
Daniel TF (1986) Systematics of Tetramerium (Acanthaceae). Syst Bot Monogr 12:1–134
Daniel TF, McDade LA, Manktelow M, Kiel CA (2008) The “Tetramerium lineage” (Acanthaceae: Acanthoideae: Justicieae): delimitation and intra-lineage relationships based on cp and nrITS sequence data. Syst Bot 33:416–436
Daniel TF, Acosta-Castellanos S (2003) Flora del Bajío y de Regiones Adyacentes. ACANTHACEAE Fascículo 117 (noviembre) México, DF
Dias-Machado C, Pereira dos Santos VL, Schimandeiro Novak R, Schechtel Koch M, Arcaro G, Raman V, Cavichiolo Franco CR, Farago PV, Manfron Budel J (2021) Contributions of trichome micromorphology to the characterization of species traded as “BOLDO.” Flora 279:151827. https://doi.org/10.1016/j.flora.2021.151827
Dörken VM, Ladd PG, Parsons RF (2023) Convergent morphology and anatomy in the microphyllous leaves of selected heathland Myrtaceae and Asteraceae. Trees 37:1225–1247
Ehleringer JR, Mooney HA (1978) Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia 37:183–200
Esau K (1977) Anatomy of seed plants. Wiley, New York
Fernandes MC, Krishnan S (2019) Anatomical characterization of Strobilanthes (Acanthaceae) species from the northern Western Ghats of India and its implication for identification at vegetative state. Nord J Bot 11:1–20
Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF (2015) Evolutionary history of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium. Int J Plant Sci 176:770–790
Gabel NH, Wise RR, Rogers GK (2021) Distribution of cystoliths in the leaves of Acanthaceae and its effect on leaf surface anatomy. Blumea 65:224–232
Gal A, Hirsch A, Siegel S, Li C, Aichmayer B, Politi Y, Fratzl P, Weiner S, Addadi L (2012) Plant cystoliths: a complex functional biocomposite of four distinct silica and amorphous calcium carbonate phases. Chem Eur J 18:10262–10270
Gul S, Ahmad M, Zafar M, Bahadur S, Sultana S, Ashfaq S, Ullah F, Kilic O, Hassan F, Siddiq Z (2018) Foliar epidermal anatomy of Lamiaceae with special emphasis on their trichomes diversity using scanning electron microscopy. Microsc Res Tech 82:206–223
Gul S, Ahmad M, Zafar M, Bahadur S, Celep F, Sultana S, Begum N, Hanif U, Zaman W, Shuaib M, Ayaz A (2019) Taxonomic significance of foliar epidermal morphology in Lamiaceae from Pakistan. Microsc Res Tech 82:1507–1528
Guo J, Zhou C (2018) Secretory structures of Pogostemon auricularius: morphology, development, and histochemistry. Symmetry 11:13. https://doi.org/10.3390/sym11010013
Inamdar JA, Chaudhari GS, Rao TR (1990) Studies on the cystoliths of Acanthaceae. Feddes Repert 101:417–424
Javelle M, Vernoud V, Rogowsky PM, Ingram GC (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189:17–39
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company Inc, New York
Kai N, Okazaki M (2003) Physiological role of plant cystolith: its possible role in suppressing the pH increase coupled with nitrate reduction in the leaves. Bull Tokyo Gakugei Univ 55:201–212
Karaismailoğlu MC, Güner Ö (2021) Trichome micromorphology of the genus Stachys sect. Fragilicaulis subsect. Fragilis and its taxonomic implications. Plant Biosyst 155:833–847
Kiel CA, Daniel TF, McDade LA (2018) Phylogenetics of new World ‘justicioids’ (Justicieae: Acanthaceae): major lineages, morphological patterns, and widespread incongruence with classification. Syst Bot 43:459–484
Kim HJ, Seo E, Kim JH, Cheong H, Kang BC, Choi D (2012) Morphological classification of trichomes associated with possible biotic stress resistance in the genus Capsicum. Plant Pathol J 28:107–113
Koch K, Ensikat HJ (2008) The hydrophobic coatings of plant surfaces: epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 39:759–772
Koch K, Blecher IC, König G, Kehraus S, Barthlott W (2009) The superhydrophilic and superoleophilic leaf surface of Ruellia devosiana (Acanthaceae): a biological model for spreading of water and oil on surfaces. Funct Plant Biol 36:339–350
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Laguna-Hernández G, Río-Zamorano CA, Meneses-Ochoa IG, Brechú-Franco AE (2017) Histochemistry and immunolocalisation of glucokinin in antidiabetic plants used in traditional Mexican medicine. Eur J Histochem 61:2782. https://doi.org/10.4081/ejh.2017.2782
Lande SK (2017) Foliar epidermal studies on some Barleria Linn., species (Acanthaceae). Indian J L Sci 6:7–12
Larcher L, Boeger MRT (2006) Anatomia foliar de Odontonema strictum (Nees) O. Kuntze (Acanthaceae). Biotemas 19:23–31
McDade LA, Daniel TF, Kiel CA (2018) The Tetramerium lineage (Acanthaceae, Justicieae) revisited: phylogenetic relationships reveal polyphyly of many New World genera accompanied by rampant evolution of floral morphology. Syst Bot 43:97–116
McKown AD, Dengler NG (2007) Key innovations in the evolution of Kranz anatomy and C4 vein pattern in Flaveria (Asteraceae). Am J Bot 94:382–399
Metcalfe CR, Chalk L (1965) Anatomy of the dicotyledons: leaves, stem, and wood in relation to taxonomy with notes on economic uses (No. 583 M588 1965). Clarendon
Muravnik LE, Kostina OV, Zaporozhets NL (2022) Structure and functions of the glandular trichomes in three Arnica species (Asteraceae), depending on their location on leaves and flowers. Flora 290:152047. https://doi.org/10.1016/j.flora.2022.152047
Naidoo Y, Kasim N, Heneidak S, Nicholas A, Naidoo G (2013) Foliar secretory trichomes of Ocimum obovatum (Lamiaceae): micromorphological structure and histochemistry. Plant Syst Evol 299:873–885
Naidoo Y, Dladla T, Dewir YH, Gangaram S, Naidoo CM, Rihan HZ (2021) The Micromorphology and Histochemistry of foliar mixed indumentum of Leucas lavandulaefolia (Lamiaceae). Plants 10:1767. https://doi.org/10.3390/plants10091767
Nawrath C (2002) The biopolymers cutin and suberin. Arabidopsis Book/ASPB 34:1–14
Nitta I, Kida A, Fujibayashi Y, Katayama H, Sugimura Y (2006) Calcium carbonate deposition in a cell wall sac formed in mulberry idioblasts. Protoplasma 228:201–208
Nurul-Aini CAC, Noraini T, Latiff A, Amirul-Aiman AJ, Ruzi AR, Idris S (2014) Taxonomic significance of leaf micromorphology in some selected taxa of Acanthaceae (Peninsular Malaysia). AIP Conf Proc 1614:727–733
Okamoto F, Rodella RA (2006) Mulberry leaf morphological, anatomical and bromatological characteristics in relation to silkworm preferences. Pesqui Agropecu Bras 41:195–203
Patil AM, Patil DA (2011) Investigations on foliar epidermal characteristics in some Acanthaceae. Curr Bot 2:1–8
Payne WW (1978) A glossary of plant hair terminology. Brittonia 30:239–255
Pierantoni M, Tenne R, Brumfeld V, Kiss V, Oron D, Addadi L, Weiner S (2017) Plants and light manipulation: the integrated mineral system in okra leaves. Adv Sci 4:1600416
Pierantoni M, Tenne R, Rephael B, Brumfeld V, van Casteren A, Kupczik K, Oron D, Addadi L, Weiner S (2018) Mineral deposits in Ficus leaves: morphologies and locations in relation to function. Plant Physiol 176:1751–1763
POWO database Website. https://powo.science.kew.org/taxon/3270802?_gl=1*1h5dg9e*_ga*MTQ0MDQwMTM1Ni4xNTg4NzA1MzEx*_ga_ZVV2HHW7P6*MTY5NjUzOTMwNi40My4xLjE2OTY1MzkzOTEuMC4wLjA.#children
Raza J, Ahmad M, Zafar M, Yaseen G, Sultana S, Majeed S (2022) Systematic significance of seed morphology and foliar anatomy among Acanthaceous taxa. Biologia 77:3125–3142
Sage TL, Busch FA, Johnson DC, Friesen PC, Stinson CR, Stata M, Sultmanis S, Rahman BA, Rawsthome S, Sage RF (2013) Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiol 163:1266–1276
Setoguchi H, Okazaki M, Suga S (1989) Calcification in higher plants with special reference to cystoliths. Origin, evolution, and modern aspects of biomineralization in plants and animals. Springer, Boston, MA, pp 409–418
Shepherd T, Wynne Griffiths D (2006) The effects of stress on plant cuticular waxes. New Phytol 171:469–499
Sreemadhavan CP, Henry AN, Subramanyam K (1968) Descriptive terminology for cystolith and raphid bearing plant organs. Taxon 17:17–18
Stevens PF (2001) Angiosperm phylogeny website. Version 14, July 2017 [and more or less continuously updated since]." will do. http://www.mobot.org/MOBOT/research/APweb/
Steyn HM, Van Wyk AE (2021) Taxonomic significance of trichomes in the genus Acanthopsis Harv. (Acanthaceae, tribe Acantheae). Adansonia 43:163–176
Takeda H, Ito F, Yamanaka S, Takiyama N, Yoshino K (2013) Roles of trichomes with silica particles on the surface of leaves in Aphananthe aspera. AIP Adv 3:032120. https://doi.org/10.1063/1.4794958
Tripp EA, Fatimah S (2012) Comparative anatomy, morphology, and molecular phylogenetics of the African genus Satanocrater (Acanthaceae). Am J Bot 99:967–982
Tripp EA, Fekadu M (2014) Comparative leaf and stem anatomy in selected species of Ruellieae (Acanthaceae) representative of all major lineages. Kew Bull 69:9543. https://doi.org/10.1007/S12225-014-9543-8
Tropicos database Website. https://www.tropicos.org
Watts S, Kariyat R (2021) Morphological characterization of trichomes shows enormous variation in shape, density and dimensions across the leaves of 14 Solanum species. AoB Plants 13:plab071. https://doi.org/10.1093/aobpla/plab071
Zhao H, Xiao MH, Zhong Y, Wang YQ (2022) Leaf epidermal micromorphology of Zingiber (Zingiberaceae) from China and its systematic significance. PhytoKeys 190:131–146
Zolla C, Argueta A (2009) Biblioteca digital de la medicina tradicional mexicana. Landsteiner Scientific, Comisión Nacional para el Desarrollo de los Pueblos Indígenas, Programa Universitario México Nación Multicultural, Universidad Nacional Autónoma de México. http://www.medicinatradicionalmexicana. unam. mx/index. php
Acknowledgements
The authors thank the following academic staff of the UNAM Faculty of Sciences: Ana Isabel Bieler-Antolin for camera and microscope assistance, Ricardo García Sandoval for edition of figures, Susana Olivares Ventura and Argelia Díaz Rico for support in the bibliographic search.
Funding
The authors developed this work with funding granted by the Faculty of Sciences of the National Autonomous University of Mexico, where we work.
Author information
Authors and Affiliations
Contributions
AEBF and GLH conceived the idea and, together with RMFJ, helped in design of the work, interpretation of the results, their discussion and manuscript writing and editing. AVM research on reports about the plant, establishment of the histochemical techniques, was part of Bachelor Thesis to obtain the title of Biologist: SEM scanning electron microscope assistance, image analysis and interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that there were no conflicts of interest involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brechú-Franco, A.E., Laguna-Hernández, G., Velázquez-Mondragón, A. et al. Structural characteristics of the leaves of two species of Tetramerium an endemic to Mexico. Braz. J. Bot 47, 205–217 (2024). https://doi.org/10.1007/s40415-024-00980-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-024-00980-6