Abstract
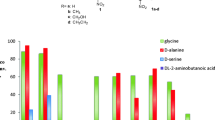
Aromaticl-amino acid transaminase is an enzyme that is able to transfer the amino group froml-glutamate to unnatural aromatic α-keto acids to generate α-ketoglutarate and unnatural aromaticl-amino acids, respectively. Enrichment culture was used to isolate thermophilicBacillus sp. T30 expressing this enzyme for use in the synthesis of unnaturall-amino acids. The asymmetric syntheses ofl-homophenylalanine andl-phenylglycine resulted in conversion yields of >95% and >93% from 150 mM 2-oxo-4-phenylbutyrate and phenylglyoxylate, respectively, usingl-glutamate as an amino donor at 60°C. Synthesizedl-homophenylalanine andl-phenylglycine were optically pure (>99% enantiomeric excess) and continuously pre-cipitated in the reaction solution due to their low solubility at the given reaction pH. While the solubility of the α-keto acid substrates is dependent on temperature, the solubility of the unnaturall-amino acid products is dependent on the reaction pH. As the solubility difference between substrate and product at the given reaction pH is therefore larger at higher temperature, the thermophilic transaminase was successfully used to shift the reaction equilibrium toward rapid product formation.
Similar content being viewed by others
References
Taylor, P. P., D. P. Pantaleone, R. F. Senkpeil, and I. G. Fotheringham (1998) Novel biosynthetic approaches to the production of unnatural amino acids using transaminases.Trends Biotechnol. 16: 412–418.
Park, H. G., J. H. Do, and H. N. Chang (2003) Regioselective enzymatic acylation of multi-hydroxyl compounds in organic synthesis.Biotechnol. Bioprocess Eng. 8: 1–8.
Krapcho, J., C. Turk, D. W. Cushman, J. R. Powell, J. M. Deforrest, E. R. Spitzmiller, D. S. Karanewsky, M. Duggan, G. Rovansak, J. Schwartz, S. Natarajan, J. D. Godfrey, D. E. Ryono, R. Neubeck, K. S. Atwal, and E. W. Petrillo (1988) Angiotensin-converting enzyme inhibitors. Mercaptan, carboxyalkyl dipeptide, and phosphinic acid inhibitors incorporating 4-substituted prolines.J. Med. Chem. 31: 1148–1160.
Lesson, P. A., X. Rabasseda, and J. Castaner (1997) FK-888.Drugs Future 22: 353–358.
Ager, D. J., I. G. Fotheringham, S. A. Laneman, D. P. Pantaleone, and P. P. Taylor (1997) The large scale synthesis of unnatural amino acids.Chim. Oggi. 15: 11–14.
Cho, B.-K., H. J. Cho, S.-H. Park, H. Yun, and B.-G. Kim (2003) Simultaneous synthesis of enantiomerically pure (S)-amino acids and (R)-amines using coupled transaminase reactions.Biotechnol. Bioeng. 81: 785–789.
Schulz, A., P. Taggeselle, D. Tripier, and K. Bartsch (1990) Stereospecific production of the herbicide phophinothricin (glufosinate) by transamination: isolation and characterization of a phosphinothricin-specific transaminase fromEscherichia coli.Appl. Environ. Microbiol. 56: 1–6.
Meiwes, J., M. Schudok, and G. Kretzschmar (1997) Asymmetric synthesis ofl-thienylalanines.Tetrahedron Asym. 8: 827–836.
Asano, Y., A. Yamada, Y. Kato, K. Yamaguchi, Y. Hibino, K. Hirai, and K. Kondo (1990) Enantioselective synthesis of (S)-amino acids by phenylalanine dehydrogenase fromBacillus sphaericus: use of natural and recombinant enzymes.J. Org. Chem. 55: 5567–5571.
Xu, Q., G. Wang, X. Wang, T. Wu, X. Pan, A. S. C. Chan, and T. K. Yang (2000) The synthesis of L-(+)-homophenylalanine hydrochloride.Tetrahedron Asym. 11: 2309–2314.
Yang, Y. J., C. H. Lee, and Y. M. Koo (2004) Separation of amino acids by simulated moving bed using competitive Langmuir isotherm.Biotechnol. Bioprocess Eng. 9: 331–338.
Ahn, J., J. Ryu, H. Jang, and J.-K. Jung (2004) Effect of growth rate on the production ofl-proline in the fed-batch culture ofCorynebacterium acetoacidophilum.Biotechnol. Bioprocess Eng. 9: 326–329.
Syldatk, C., D. Völkel, U. Bilitewski, K. Krohn, H. Höke, and F. Wagner (1992) Biotechnological production of unnaturall-amino acids fromD,L-5-monosubstituted hydantions. II.l-α- andl-β-naphthylalanine.Biotechnol. Lett. 14: 105–110.
Cooper, A. J. L., J. Z. Ginos, and A. Meister (1983) Synthesis and properties of the β-keto acids.Chem. Rev. 83: 321–358.
Cho, B. K., J. H. Seo, T. W. Kang, and B. G. Kim (2003) Asymmetric synthesis ofl-homophenylalanine by equilibrium-shift using recombinant aromaticl-amino acid transaminase.Biotechnol. Bioeng. 83: 226–234.
Shin, J.-S., and B.-G. Kim (2002) Exploring the active site of amine: pyruvate aminotransferase on the basis of the substrate structure-reactivity relationship: How the enzyme controls substrate specificity and stereoselectivity.J. Org. Chem. 67: 2848–2853.
Peisach, D., D. M. Chipman, P. W. Van Ophem, J. M. Manning, and D. Ringe (1998) Crystallographic study of steps along the reaction pathway of D-amino acid aminotransferase.Biochemistry 37: 4958–4967.
Stewart, J. D. (2001) Dehydrogenases and transaminases in asymmetric synthesis.Curr. Opin. Chem. Biol. 5: 120–129.
Chao, Y. P., Z. J. Lai, P. Chen, and J. T. Chern (1999) Enhanced conversion rate ofl-phenylalanine by coupling reactions of aminotransferases and phosphoenolpyruvate carboxykinase inEscherichia coli K-12.Biotechnol. Prog. 15: 453–458.
Fotheringham, I. G., N. Grinter, D. P. Pantaleone, R. F. Senkpeil, and P. P. Taylor (1999) Engineering of a novel biochemical pathway for the biosynthesis ofl-2-aminobutyric acid inEscherichia coli K-12.Bioorg Med. Chem. 7: 2209–2213.
Cho, B.-K., H. J. Cho, H. Yun, and B.-G. Kim (2003) Simultaneous synthesis of enantiomerically pure (S)-amino acids and (R)-amines using α/β-amino transferase coupling reactions with two-liquid phase reaction system.J. Mol. Catal., B Enzym. 26: 273–285.
Lo, H.-H., S.-K. Hsu, W.-D. Lin, N.-L. Chan, and W.-H. Hsu (2003) Asymmetrical synthesis ofl-homophenylalanine using engineeredEscherichia coli aspartate aminotransferase.Biotechnol. Prog. 21: 411–415.
Twomey, C. M. and S. Doonan (1997) A comparative study of the thermal inactivation of cytosol and mitochondrial aspartate aminotransferase.Biochim. Biophys. Acta 1342: 37–44.
Zale, S. E., and A. M. Klibanov (1983) On the role of reversible denaturation (unfolding) in the irreversible thermal inactivation of enzymes.Biotechnol. Bioeng. 25: 2221–2230.
Cho, B.-K., H.-Y. Park, J.-H. Seo, K. Kinnera, B.-S. Lee, and B.-G. Kim (2004) Enzymatic resolution for the preparation of enantiomerically enriched D-β-heterocyclic alanine derivatives usingEscherichia coli aromaticl-amino acid transaminase.Biotechnol. Bioeng. 88: 512–519.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, BK., Seo, JH., Kim, J. et al. Asymmetric synthesis of unnaturall-amino acids using thermophilic aromaticl-amino acid transaminase. Biotechnol. Bioprocess Eng. 11, 299–305 (2006). https://doi.org/10.1007/BF03026244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03026244