Abstract
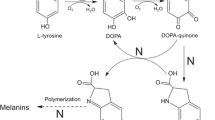
Purified frog epidermis tyrosinase was immobilized on the following supports: CNBr-Sepharose 4B, Enzacryl AA, Enzacryl AH, Enzacryl Polythiolactone, and Enzacryl Polyacetal. The enzyme was active on all supports except when Enzacryl Polyacetal was used. The stability increased on immobilization. Enzacryl AA was the best support assayed. The Enzacryl AA-enzyme complex was 30- to 40-fold more stable to inactivation reaction than soluble enzyme, and maintained its activity when stored and assayed repeatedly. The immobilized enzyme on the other supports was also more stable than the soluble form. The pH-activity profile, thermal stability, storage stability, and the effect of protein concentration on activity of the immobilized enzyme have been studied. The properties observed for the immobilized enzyme were different than those of the soluble enzyme. The main reason for this difference could be due to enzyme modification through tyrosine groups of the enzyme; to conformational changes produced in the union to the matrix; and to microenvironmental differences created by the matrix.
Similar content being viewed by others
References
Enzyme Nomenclature: Commission on Biochemical Nomenclature (1973) Elsevier, Amsterdam.
Nicolaus, R. A. (1968). Melanins, 2nd ed., Hermann, Paris.
McGuire, J. S. (1970) Biochem. Biophys. Res. Commun. 40: 1084.
Nelson, J. M. andDawson, C. R. (1944) Adv. Enzymol. 4:99.
Wykes, J. R., Dunnill, P. andLilly, M. D. (1971) Nature New Biol. 230:181.
Letts, D. andChase, Th. (1974) Adv. Exp. Med. Biol. 42:317.
Wood, B. J. B. andIngraham, L. L. (1965) Nature 205:29.
Shinao, K., Seiji, M., andFukuzawa, H. (1974) Tohoku J. Exp. Med. 114:263.
Iborra, J. L., Cortés, E., Manjón, A., Ferragut, J. A., andLlorca, F. I. (1976) J. Solid-Phase Biochem. 1:91.
Hartree, E. (1972) Anal. Biochem. 48:422.
Padrón, M. P., Lozano, J. A., andGonzález, A. G. (1975) Phytochemistry 14:1959.
Goldstein, L., Petch, M., Blumberg, S., Atlas, D., andLevin, Y. (1970) Biochemistry 9:2322.
Axen, R., andErnback, S. (1971) Eur. J. Biochem. 18:351.
Schell, H. D., andMateescu, M. A. (1975) Acta Biol. Med. Germ. 34:959.
Zaborsky, O. R. (1973) Immobilized Enzymes,Weast, R. C. (ed.), CRC Press, Cleveland, Ohio.
Chen, Y. M. (1975) J. Invest. Dermatol. 64:77.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iborra, J.L., Manjon, A. & Lozano, J.A. Stability of immobilized frog epidermis tyrosinase. Journal of Solid-Phase Biochemistry 2, 85–96 (1977). https://doi.org/10.1007/BF02991398
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02991398