Abstract
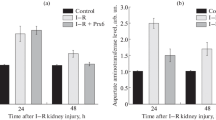
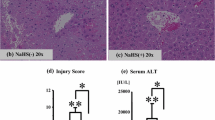
The focus of this study was to investigate the influences of enzymatic scavengers of active oxygen metabolites and phospholipase A2 inhibitor on hepatic secretory and microsomal function during hepatic ischemia/reperfusion. Rats were pretreated with free radical scavengers such as superoxide dismutase (SOD), catalase, deferoxamine and phospholipase A2 inhibitor such as quinacrine and then subjected to 60 min. no-flow hepatic ischemiain vivo. After 1, 5 hr of reperfusion, bile was collected, blood was obtained from the abdominal aorta, and liver microsomes were isolated. Serum aminotransferase (ALT) level was increased at 1 hr and peaked at 5 hr. The increase in ALT was significantly attenuated by SOD plus catalase, deferoxamine and quinacrine especially at 5 hr of reperfusion. The wet weight-to-dry weight ratio of the liver was significantly increased by ischemia/reperfusion. SOD and catalase treatment minimized the increase in this ratio. Hepatic lipid peroxidation was elevated by ischemia/reperfusion, and this elevation was inhibited by free radical scavengers and quinacrine. Bile flow and cholate output, but not bilirubin output, were markedly decreased by ischemia/reperfusion and quinacrine restored the secretion. Cytochrome P450 content was decreased by ischemia/reperfusion and restored by free radical scavengers and quinacrine to the level of that of the sham operated group. Aminopyrine N-demethylase activity was decreased and anilinep-hydroxylase was increased by ischemia/reperfusion. The changes in the activities of the two enzymes were prevented by free radical scavengers and quinacrine. Our findings suggest that ischemia/reperfusion diminishes hepatic secretory functions as well as microsomal drug metabolizing systems by increasing lipid peroxidation, and in addition to free radicals, other factors such as phospholipase A2 are involved in pathogenes of hepatic dysfunction after ischemia/reperfusion.
Similar content being viewed by others
References cited
Astrom, A. and DePierre, J. W., Rat liver microsomal cytochrome P-450: purification, characterization, multiplicity and induction.Biochem. Biophys. Acta., 853, 1–27 (1986).
Atalla, S. L., Toledo-Pereyra, L. H., Mackenzie, G. H. and Cederna, J. P., Influence of oxygen-derived free radical scavengers on ischemic livers.Transplantation, 40, 584–590 (1985).
Bast, A., Is formation of reactive oxygen by cytochrome P-450 perilous and predictable.Trends Pharmacol. Sci., 7, 266 (1986).
Benveniste, J., Chignard, M., Le Couedic, J. and Vargaftig, B., Biosynthesis of platelet-activating factor. II. Involvement of phospholipase A2 in the formation of PAF-acether and lyso PAF-acether from rabbit platelets.Throm. Res., 25, 375–385 (1982).
Bromont, C., Marie, C. and Bralet, J., Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats.Stroke, 20, 918–924 (1989).
Drugas, G. T., Paidas, C. N., Yahanda, A. M., Ferguson, D. and Clemens, M. G., Conjugated deferoxamine attenuates hepatic microvascular injury following ischemia/reperfusion.Circ. Shock, 34, 278–283 (1991).
Granger, D. N., Hollwarth, M. E. and Parks, D. A., Ischemia-reperfusion injury: role of oxygen derived free radical.Acta. Physiol. Scan., 548, 47–63 (1986).
Hogberg, J., Bergstrand, A. and Jakobsson, S. V., Lipid peroxidation of rat liver micro-somes.Eur. J. Biochem., 37, 51–59 (1973).
Hrycay, E. G. and O'brien, P., The peroxidase nature of cytochrome P420 utilizing a lipid peroxide substrate.Arch. Biochem. Biophys., 147, 28–35 (1971).
Irvin, J. L., Johnston, C. G. and Kopara, J., A photometic method for the determination of cholate in bile and blood.J. Biol. Chem., 153, 439–450 (1944).
Kawase, T., Kato, S. and Lieber, C. S., Lipid peroxidation and antioxidant defense system in rat liver after chronic ethanol feeding.Hepatology, 10, 815–821 (1989).
Lee, S. M. and Clemens, M. G., Effect of α-to-copherol on hepatic mixed function oxidase in hepatic ischemia/reperfusion.Hepatology, 15, 276–281 (1992).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J., Protein measurement with folin phenol reagent.J. Biol. Chem., 193, 265–275 (1951).
Masugi, F., and Nagamura, T., Effect of vitamin E deficiency on the level of superoxide dismutase, glutathione peroxidase, catalase and lipid peroxide in the rat liver.Int. J. Vitam. Nutr. Res., 46, 187–191 (1976).
Mead, J. F., Free radicals in biology, New York,Academic Press, 1979, pp. 51–70.
Mieyal, J. and Blumer, J., Acceleration of the autooxidation of human oxyhemoglobin by aniline: its relation to hemoglobin-catalyzed aniline hydroxylation.J. Biol. Chem., 251, 3442–3445 (1976).
Omar, R., Nomilos, I., Piccorelli, G., Savino, G. and Agarwal, N., Prevention of post ischemic lipid peroxidation and liver cell injury by iron chelation.Gut, 30, 510–514 (1989).
Omura, T. and Sato, R., The carbon monoxide-binding pigment of liver microsome.J. Biol. Chem., 239, 2370–2378 (1964).
Otamiri, T., Lindmark, D., Franzen, L. and Tagesson C., Increased phospholipase A2 and decreased lysophospholipase activity in the small intestinal mucosa after ischemia and revasculization.Gut, 28, 1445–1453 (1987).
Otamiri, T. and Tagesson, C., Role of phospholipase A2 and oxygen free radicals in mucosal damage after small intestinal ischemia and reperfusion.Am. J. Surg., 157, 562–566 (1989).
Parks, D. A. and Granger, D. N., Contribution of ischemia and reperfusion to mucosal lesion formation.Am. J. Physiol., 250, G749-G753 (1986).
Petty, M., Grisar, J. M., Dow, J. and Jong, W. D., Effects of an α-tocopherol analogue on myocardial ischemia and reperfusion injury in rats.Eur. J. Pharmacol., 179, 241–242 (1991).
Schenkman, J. B., Remmer, H. and Estrabook, R. W., Spectral studies of drug interaction with hepatic microsomal cytochrome.Mol. Pharmacol., 3, 113–123 (1967).
Sevanian, A., Muakkassah-Kelly, S. and Montestrugue, S., The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxidation.Arch. Biochem. Biophys., 223, 441–452 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Park, MJ., Cho, TS. & Lee, SM. The role of oxygen free radicals and phospholipase A2 in ischemia-reperfusion injury to the liver. Arch. Pharm. Res. 18, 189–194 (1995). https://doi.org/10.1007/BF02979194
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02979194