Abstract
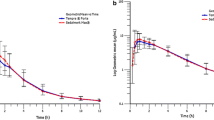
Acetaminophen (AAP) and ranitidine (RT) were coadministered orally to nine rats, and the possible contribution of the gastric emptying to the plasma concentration profiles of them was examined. The drugs showed multiple plasma peaks similar to the respective ones after separated administration of each durg. It implies that there is no significant interaction between AAP and RT in terms of the gastric emptying or drug absorption. There were no significant linear correlations of the peak patterns (peak height and peak time) between AAP andd RT. It is contrary to the expectation from the biphasic gastric emptying (BGE) theory previously suggested for AAP and RT. The BGE theory, therefore, seemed to have some draw-backs in explaining satisfactorily the multiple plasma peaks of AAP and RT. Two more doubts raised previously against the BGE theory were also discussed.
Similar content being viewed by others
Literature Cited
Clement, J. A., Heading, R. C., Nimmo, W. S. and Prescott, L. F., Kinetics of acetaminophen absorption and gastric emptying in man.Clin, Pharmacol. Ther.,24, 420–431 (1978).
Oberle, R. L. and Amidon, G. L.: The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine: An explanation for the double peak phenomenon.J. Pharmacokin. Biopharm. 15, 529–544 (1987).
Shim, C. K., Kim M. A., Lee, M. H. and Kim, S. K., Development of controlled release oral drug delivery systems by membrane-coating technique II. Correlation between saliva and plasma concentration of acetaminophenin man.J. Kor. Pharm. Sci.,20, 29–33 (1990).
Shim, C. K. and Jung, B. H.: Inter-and intrasubject variations of multiple saliva peaks of acetaminophen after oral administration of tablest.Int. J. Pharmaceut.,82, 233–237 (1992).
Shim, C. K. and Jung, B. H.: Noncontribution of enterohepatic recycling to multiple plasma peaks of acetaminphen after oral administration to rats.Int. J. Pharmaceut. 83, 257–262 (1992).
Shim, C. K. and Hong, J. S.: Inter-and intrasubject variation of ranitidine pharmacokinetics after oral administration to normal male subjects.J. Pharm. Sci.,78, 990–94 (1989).
Shim, C. K. and Kim, M. S.: Mechanism of multiple plasma peak phenomenon of ranitidine after oral administration to rats. MS thesis, Seoul National University p. 42–48 (1991).
Miller, R.: Pharmacokinetics and bioavailability of ranitidine in humans.J. Pharm. Sci.,73, 1376–1379 (1984).
Bodemar, G., Norlander, B., Fransson, L. Walan, A.: The absorption of cimetidine before and during maintenance treatment with cimetidine and the influence of a meal on the absorption of cimetidine-Studies in patients with peptic ulcer disease.Br. J. Clin. Pharmacol.,7, 23–31 (1979).
Griffiths, R., Lee, R. M and Taylor, D. C.: Kinetics of cimetidine in man and experimental animals, In Burland, W. L. and Simkins, M. A. (ed),Proceedings of the Second International Symposium on Histamine H 2-Receptor Antagonists. Extra. Medica, Amsterdam. pp. 38–59 (1977).
Chungi, V. S., Dittert, L. W. and Smith, P. B.: Gastrointestinal sites of furosemide absorption in rats.Int. J. Pharmaceut.,4, 27–38 (1979).
Waller, E. S., Hamilton, S. F., Massarella, J. W., Sharanevych, M. A., Smith, R. V., Yakatan, G. J. and Dolusio, J. T.: Disposition and absolute bioavailability of furosemide in healthy males.J. Pharm. Sci.,71, 1105–1108 (1982).
Staveris, S., Houin, G., Tillement, J. P., Jamet, G., Schneider, M., Jung, L. and Koffel, J. C.: Primary dose-dependent pharmacokinetic study of veralipride.J. Pharm. Sci.,74, 94–96 (1985).
Plusquellec, Y., Campistron, G., Staveris, S., Barre, J., Jung, L., Tillement, J. P. and Houin, G.: A double-peak phenomenon in the pharmacokinetics of veralipride after oral administration: A double-site model for drug absorption.J. Pharmacokin. Biopharm. 15, 225–239 (1987).
Brockmeier, D., Grigoleit, H. G. and Leonhardt, H.: The absorption of piretanide from the gastro-intestinal tract is site-dependent.Eur. J. Clin. Pharmacol.,30, 79–82 (1986).
Funaki, T., Furuta, S. and Kaneniwa, N.: Discontinuous absorption property of cimetidine.Int. J. Pharmaceut.,31, 119–123 (1986).
Murata, K., Noda, K., Kohno, K. and Samejima, M.: Pharmacokinetic analysis of concentration data of drugs with irregular absorption profiles using multi-fraction absorption models.J. Pharm. Sci. 76, 109–113 (1987).
Murata, K., Tagawa, K., Noda, K. and Samejima, M.: Pharmacokinetic analysis of single-or multiple-dose plasma drug concentration data with a microcomputer using multi-fraction absorption models.J. Pharm. Sci. 78, 154–159 (1989).
Pedersen, P. V. and Miller, R.: Pharmacokinetics and bioavailability of cimetidine in humans.J. Pharm. Sci.,69, 394–398 (1980).
Pedersen, P. V.: Pharmacokinetic analysis of linear system approach I: Cimetidine bioavailability and second peak phenomenon.J. Pharm. Sci. 70, 32–38 (1981).
Shim, C. K. and Lee, H. W.: Mechanism of multiple plasma peak of oral ranitidine-presumption of biphasic gastric emptying pattern.Abstracts of the 20th Annual Academic Convention of The Korean Society of Pharmaceutics, Seoul, November 23–24, 1990.
Ziemniak, J. A., Welage, L. S. and Schentag, J. J.: In: W. E. Evans, J. J., Schentag and W. J. Jusko (Ed.),Applied Pharmacokinetics, Principle of Therapeutic Drug Monitoring, 2nd ed. Applied Therapeutics Inc., Spokane, W. A., pp. 782–786 (1986).
Lin, J. H. and Levy, G.: Effect of experimental renal failure on sulfate retention and acetaminophen pharmacokinetics in rats.J. Pharmacol. Exp. Therap. 221, 80–84 (1982).
Lebert, P. A., MacLeod, S. M., Mahon, W. A., Soldin, S. J. and Vadenberghe, H. M.: Ranitidine kinetics and dynamics. I. oral dose studies.Clin. Pharmacol. Ther. 30, 770–774 (1981).
Mojaverian, P., Reynolds, J. C., Ouyang, A., Wirth, F., Kellner, P. E. and Vlasses, P. H.: Mechanism of gastric emptying of a nondisintegrating radiotelemetry capsule in man.Pharm. Res. 8, 97–100 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shim, CK., Suh, MK. Multiple plasma peaks of acetaminophen and ranitidine after simultaneous oral administration to rats. Arch. Pharm. Res. 15, 246–250 (1992). https://doi.org/10.1007/BF02974064
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02974064