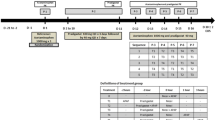
The pharmacokinetic properties of a finished dosage form (FDF) of a combination of lappaconitine, glycyrrhizic acid, and methyluracil (LGM) possessing antiarrhythmic activity were studied. The FDF of LGM upon a single intragastric administration in various doses [1680, 3360, and 6720 mg/kg in terms of lappaconitine (LC)] was rapidly absorbed from the gastrointestinal tract with the maximum blood-plasma concentration of LC being reached 15 min after drug intake. LC had a short elimination half-life and retention time in the body. The drug was extensively distributed throughout organs and tissues and exhibited tropism for the kidneys, liver, and myocardium. LC was metabolized to form N-deacetyllappaconitine (N-DLC). Its metabolite was detected in urine for at least 72 h, which indicated predominant excretion of the drug through the kidneys. The pharmacokinetic profile of N-DLC was similar to that of LC.



Similar content being viewed by others
References
L. V. Serdoz, H. Rittger, F. Furlanello, et al., Pharm. Res., 144, 257 – 263 (2019).
V. N. Stolyaruk, I. B. Tsorin, M. B. Vititnova, et al., Farmakokinet. Farmakodin., 2, 16 – 18 (2017).
V. N. Stolyaruk, I. B. Tsorin, M. B. Vititnova, et al., Farmakokinet. Farmakodin., 2, 12 – 15 (2017).
M. A. Korolev, V. I. Konenkov, L. N. Rachkovskaya, et al., Sib. Nauchn. Med. Zh., 39(6), 14 – 21 (2019).
R. M. Kataeva, E. F. Agletdinov, K. V. Bulygin, et al., Sechenov. Vestn., 10(1), 22 – 28 (2019).
MH RF Order No. 199n of Apr. 1, 2016. On Approval of Good Laboratory Practice Rules, Bulletin of Regulations of Federal Executive Organs, 37, 7 (2016).
V. K. Piotrovskii, Farmakol. Toksikol., 49(5), 118 – 127 (1986).
M. S. Yunusov, Yu. I. Murinov, et al., RU Pat. No. 2,664,668, Aug. 21, 2018; Byull., No. 30 (2018).
S. Glantz, Primer of Biostatistics, 4th Ed., McGraw-Hill Inc., New York (1997), 473 pp.
Acknowledgments
The article was written using results from preclinical studies of the drug based on a combination of LC, glycyrrhizic acid, and methyluracil. The research was conducted in the framework of State Contract for R&D No. 14.N08.11.0068 of Dec. 2, 2015 and was directed by Prof. V. A. Kataev, Chair of the Pharmacy Department, BSMU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 6, pp. 3 – 7, June, 2021.
Rights and permissions
About this article
Cite this article
Kataev, V.A., Petrova, S.F., Perfilova, V.N. et al. Pharmacokinetic Parameters of the Lappaconitine, Glycyrrhizic Acid and Methyluracil Combination Exhibiting Antiarrhythmic Properties upon Single Intragastric Administration in Various Doses. Pharm Chem J 55, 531–535 (2021). https://doi.org/10.1007/s11094-021-02454-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02454-5