Abstract
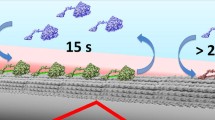
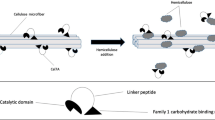
The binding of cellobiohydrolases to cellulose is a crucial initial step in cellulose hydrolysis. In the search for a detailed understanding of the function of cellobiohydrolases, much information concerning how the enzymes and their constituent catalytic and cellulose-binding changes during hydrolysis is still needed. The adsorption of purffied two cellobiohydrolases (Ce17A and Ce16A) fromTrichoderma reesei cellulase to microcrystalline cellulose has been studied. Cellobiohydrolase II (Ce16A) does not affect the adsorption of cellobiohydrolase I (Ce17A) significantly, and there are specific binding sites for both Ce17A and Ce16A. The adsorption affinity and tightness of the cellulase binding domain (CBD) for Ce17A are larger that those of the CBD for Ce16A. The CBD for Ce17A binds more rapidly and tightly to Avicel than the CBD for Ce16A. The decrease in adsorption observed when the two cellobihydrolases are studied together would appear to be the result of competition for binding sites on the cellulose. Ce17A competes more efficiently for binding sites than Ce16A. Competition for binding sites is the dominating factor when the two enzymes are acting together, furthermore adsorption to sites specific for Ce17A and Ce16A, also contributes to the total adsorption.
Similar content being viewed by others
References
Teeri, T. T. (1997) Crystalline cellulose degradation: New insights into the function of cellobiohydrolases.Trends Biotechnol. 15: 160–167.
Barr, B. K., Y. L. Hsieh, B. Ganem, and D. B. Wilson (1996) Identification of two functionally different classes of exocellulases.Biochemistry 35: 586–592.
Henrissat, S., T. T. Teeri, and R. A. Warren (1998) Scheme for designating enzymes that hydrolyse the polysaccharides in the cell walls of plants.FEBS Lett. 425: 352–354.
Woodward, J. (1991) Synorgism in cellulase systems.Bioresoures Technol. 36: 67–75.
Fagerstam, L. C. and L. G. Pettersson (1980) The 1.4-β-glucan cellobiohydrolase ofTrichoderma reesi QM 9414. A new type of cellulolytic synergism.FEBS Lett. 119: 97–100.
Tomine, P., H. van Tilbeurgh, G. Pettersson, J. van Damme, J. Vandekerckhove, J. Knowles, T. Teeri, and M. Claeyssens (1988) Studies of the cellulolytic system ofTrichodernia reesei QM 9414: Analysis of domain function in two cellobiotydrolases by limited proteolysis.Eur. J. Biochem. 170: 575–581.
Knowles, J., P. Lehtovaara, and T. Teeri (1987) Cellulase families and their genes.Trends Biotechnol. 5: 255–261.
Ong, E., J. M. Greenwood, N. R. Gilkes, D. C. Kilburn, R. C. Miller Jr, and R. A. J. Warren (1989) The cellulasebinding domains of cellulases: Tools for biotechnology.Trends Biotechnol. 7: 234–243.
Johansson, G., J. Sthlberg, G. Lindeberg, A. EngstrÖm, and G. Pettersson (1989) Isdlated fungal cellulase terminal domains and synthetic minimum analogue bind to cellulose.FEBS Lett. 243: 389–393.
Kim, D. W., Y. H. Jang, and Y. K. Jeong (1997) Adsorption behaviors of two cellobiohydrolases and their core proteins fromTrichoderma reesei on Avicel PH 101.Biotechnol. Lett. 19: 893–897.
Kotiranta, P., J. Karlsson, M. Siika-Aho, J. Medve, L. Viikari, M. Tjerneld, and M. Tenkanen (1999) Adsorption and activity ofTrichoderma reesei cellobiohydrolase I, endoglucanase II, and the corresponding core proteins on steam pretreated willow.Appl. Biochem. Biotechnol. 81: 81–90.
Kraulis, P., M. Clore, M. Nilges, A. Jones, G. Pettersson, J. Knowles, and G. Gronenboru (1989) Determination of the three-dimensional Solution structure of the Cterminal domain of celloboohydrolase I fromTrichoderma reese.Biochemistry 28: 7241–7257.
Wood, T. M. and S. I. McCrae (1986) The cellulase ofPenicilliani pittophilium.Biochem. J. 234: 93–99.
Stuart, J. Y. and D. L. Ristroph (1985) Analysis of cellulose-cellulase adsorption data: A fundamental approach.Biotechnol. Bioeng. 27: 1056–1059.
Peitersen, N., J. Medeiros, and M. Mandels (1997) Adsorption ofTrichoderma cellulase on cellulose.Biotedinol. Bioeng. 19: 1091–1094.
Bhikhabhai, R., G. Iohansson, and G. Pettersson (1984) Isolation of cellulolytic enzymes fromTrichoderma reesei QM 9414.J. Appl. Biochem. 6: 336–345.
Summer, J. B., G. F. Somer (1944)Laboratory Experiments in Biological Chemistry. pp. 34–37. Academic Press, NY, USA.
Ellouz, S., H. Durand, and G. Tiraby (1987) Analytical separation ofTrichoderma reesei: cellulase by ion exchange fast protein liquid chromatography.J. Chromatogr. 396: 307–317.
Belldman, G., A, G. J. Voragen, F. M. Rombouts, M. F. Scarle-van Leeuwen, and W. Pilnik (1987) Adsorption and kinetic behavior of purified endoglucanase and exoglucanases fromTrichoderma viride.Biotechnol. Bioeng. 30: 251–257.
Stahlberg, J., G. Johansson, and G. Pattersson (1991) A new model for enzymatic hydrolysis of cellulose based on the two-domain structure of cellobiohydrolaseBio/Technology 9: 286–290.
Reese, E. T. (1982) Elution of cellulase from cellulose.Process Biochem. 17: 2–8.
Woodward, J., M. K. Hayes, and N. E. Lee (1988) Hydrolysis of cellulose by saturation and non-saturating concentrations of cellulose: Implications for synergism.Bio/Technology 6: 301–304.
Kyriacou, A., R. J. Neufeld, and C. R. MacKenzie (1988) Effect of physical parameters on the adsorption characteristics of fractionatedTrichoderma reesei cellulase components.Enzyme Microb. Technol. 10: 675–681.
Chanzy, H., B. Henrissat, and R. Vuong (1984) Colloidal gold labeiing of 1,4-beta-D-glucan cellobiohydrolase adsorbed on cellulose substrates.FEBS Lett. 172: 193–197.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.W., Hong, Y.G. Description of cellobiohydrolases Ce16A and Ce17A fromTrichoderma reesei using Langmuir-type models. Biotechnol. Bioprocess Eng. 6, 89–94 (2001). https://doi.org/10.1007/BF02931952
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931952