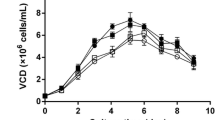
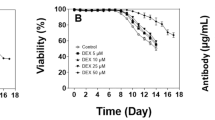
Abstract
The stabilization of optimum pH for cells can cause a higher erythropoietin (EPO) production rate and a good growth rate with the prolonged culture span in recombinant Chinese hamster ovary (r-CHO) cells. Our strategy for stabilizing the optimum pH in this study is to reduce the lactate production by adding sodium lactate to a culture medium. When 40 mM sodium lactate was added, a specific growth rate was decreased by approximately 22% as compared with the control culture. However the culture longevity was extended to 187 h, and more than a 2.7-fold increase in a final accumulated EPO concentration was obtained at 40 mM of sodium lactate. On the condition that caused the high production of EPO, a specific glucose consumption rate and lactate production rate decreased by 23.3 and 52%, respectively. Activity of lactate dehydrogenase (LDH) in r-CHO cells increased and catalyzed the oxidation of lactate to pyruvate, together with the reverse reaction, at the addition of 40 mM sodium lactate. The addition of 40 mM sodium lactate caused the positive effects on a cell growth and an EPO production in the absence of carbon dioxide gas as well as in the presence of carbon dioxide gas by reducing the accumulation of lactate.
Similar content being viewed by others
References
Park, H., S. An, and T. Choe (2006) Change of insulin-like growth factor gene expression in Chinese hamster ovary cells cultured in serum-free media.Biotechnol. Bioprocess Eng. 11: 319–324.
Kim, J. S., M. K. Min, and E. C. Jo (2001) High-level expression and characterization of single chain urokinase-type plasminogen activator (scu-PA) produced in recombinant Chinese hamster ovary (CHO) cells.Biotechnol. Bioprocess Eng. 6: 117–127.
Kato, H., T. Inoue, N. Ishii, Y. Murakami, M. Matsumura, T. Seya, and P. C. Wang (2002) A novel simple method to purify recombinant soluble human complement receptor type 1 (sCR1) from CHO cell culture.Biotechnol. Bioprocess Eng. 7: 67–75.
Chang, K. H., K. S. Kim, and J. H. Kim (1998) Analysis of erythropoietin glycoform produced by recombinant CHO cells using the lectin-blotting technique.Biotechnol. Bioprocess Eng. 3: 40–43.
Bae, G. W., D. W. Jeong, H. J. Kim, G. M. Lee, H. W. Park, T. B. Choe, S. M. Kang, I. Y. Kim, and I. H. Kim (2006) High productivity of t-PA in CHO cells using hypoxia response element.J. Microbiol. Biotechnol. 16: 695–703.
Li, J., C. Menzel, D. Meier, C. Zhang, S. Dubel, and T. Jostock (2007) A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies.J. Immunol. Methods 318: 113–124.
Choi, Y. S., D. Y. Lee, I. Y. Kim, S. Kang, K. Ahn, H. J. Kim, Y. H. Jeong, G. T. Chun, J. K. Park, and I. H. Kim (2000) Ammonia removal using hepatoma cells in mammalian cell cultures.Biotechnol. Prog. 16: 760–768.
Kim, N. Y., Y. J. Lee, H. J. Kim, J. H. Chol, J. K. Kim, K. H. Chang, J. H. Kim, and H. J. Kim (2004) Enhancement of erythropoietin production from Chinese hamster ovary (CHO) cells by introduction of the urea cycle enzymes, carbamoyl phosphate synthetase 1 and ornithine transcarbamylase.J. Microbiol. Biotechnol. 14: 845–851.
Lao, M. S. and D. Toth (1997) Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture.Biotechnol. Prog. 13: 688–691.
Omasa, T., K. Higashiyama, S. Shioya, and K. Suga (1992) Effects of lactate concentration on hybridoma culture in lactate-controlled fed-batch operation.Biotechnol. Bioeng. 39: 556–564.
Ozturk, S. S., M. R. Riley, and B. O. Palsson (1992) Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production.Biotechnol. Bioeng. 39: 418–431.
Hassell, T., S. Gleave, and M. Butler (1991) Growth inhibition in animal cell culture. The effect of lactate and ammonia.Appl. Biochem. Biotechnol. 30: 29–41.
Kurano, N., C. Leist, F. Messi, S. Kurano, and A. Fiechter (1990) Growth behavior of Chinese hamster ovary cells in a compact loop bioreactor. 2. Effects of medium components and waste products.J. Biotechnol. 15: 113–128.
Glacken, M. W., E. Adema, and A. J. Sinskey (1988) Mathematical descriptions of hybridoma culture kinetics: I. Initial metabolic rates.Biotechnol. Bioeng. 32: 491–506.
Reuveny, S., D. Velez, J. D. Macmillan, and L. Miller (1987) Factors affecting monoclonal antibody production in culture.Dev. Biol. Stand. 66: 169–175.
Miller, W. M., C. R. Wilke, and H. W. Blanch (1988) Transient responses of hybridoma cells to lactate and ammonia pulse and step changes in continuous culture.Bioprocess Eng. 3: 113–122.
Singh, R. P., M. Al-Rubeai, C. D. Gregory, and A. N. Emery (1994) Cell death by necrosis and apoptosis during the culture of commercially important cell lines. pp 187–191. In: R. E. Spier, J. B. Griffiths, and W. Berthold (eds.),Animal Cell Technology: Products of Today, Prospects for Tonorrow, Butterworth-Heinemann, Oxford, UK.
Yang, M. and M. Butler (2000) Effects of ammonia on CHO cell growth, erythropoietin production, and glycosylation.Biotechnol. Bioeng. 68: 370–380.
Porter, D. L. and M. A. Goldberg (1994) Physiology of erythropoictin production.Semin. Hematol. 31: 112–121.
Miller, M. E., M. Rorth, H. H. Parving, D. Howard, I. Reddington, C. R. Valeri, and F. Stohlman, Jr. (1973) pH effect on erythropoletin response to hypoxia.N. Engl. J. Med. 288: 706–710.
Thorens, B. and P. Vassalli (1986) Chloroquine and ammonium chloride prevent terminal glycosylation of immunoglobulins in plasma cells without affecting secretion.Nature 321: 618–620.
Glacken, M. W., R. J. Fleischaker, and A. J. Sinskey (1986) Reduction of waste product excretion via nutrient control: Possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells,Biotechnol. Bioeng. 28: 1376–1389.
Reitzer, L. J., B. M. Wice, and D. Kennell (1979) Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells.J. Biol. Chem. 254: 2669–2676.
Eagle, H., S. Barban, M. Levy, and H. O. Schulze (1958) The utilization of carbohydrates by human cell cultures.J. Biol. Chem. 233: 551–558.
Xie, L. and D. I. C. Wang (1994) Fed-batch cultivation of animal cells using different medium design concepts and feeding strategies.Biotechnol. Bioeng. 95: 270–284.
Miller, W. M., H. W. Blanch, and C. R. Wilke (1988) A kinetic analysis of hybridoma growth and metabolism in batch and continuous culture: effect of nutrient concentration, dilution rate, and pH.Biotechnol. Bioeng. 32: 947–965.
Ozturk, S. S. and B. O. Palsson (1991) Growth, metabolic, and antibody production kinetics of hybridoma cell culture: I. Analysis of data from controlled batch reactors.Biotechnol. Prog. 7: 471–480.
Chiba, S., F. Takaku, T. Tange, K. Shibuya, C. Misawa, K. Sasaki, K. Miyagawa, Y. Yazaki, and H. Hirai (1991) Establishment and erythroid differentiation of a cytokine-dependent human leukemic cell line F-36E: A parental line requiring granulocyte-macrophage colonystimulating factor or interleukin-3, and a subline requiring erythropoietin.Blood 78: 2261–2268.
Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays.J. Immunol. Methods 65: 55–63.
Donnelly, M. and I. E. Scheffler (1976) Energy metabolism in respiration-deficient and wild type Chinese hamster fibroblasts in culture.J. Cell Physiol. 89: 39–51.
Kimura, R. and W. M. Miller (1996) Effects of elevated pCO2 and/or osmolality on the growth and recombinant tPA production of CHO cells.Biotechnol. Bioeng. 52: 152–160.
Madshus, I. H. (1988) Regulation of intracellular pH in eukaryotic cells.Biochem. J. 250: 1–8.
Gramer, M. J. and C. F. Goochee (1993) Glycosidase activities in Chinese hamster ovary cell lysate and cell culture supernatant.Biotechnol. Prog. 9: 366–373.
Cruz, H. J., C. M. Freitas, P. M. Alves, J. L. Moreira, and M. J. T. Carrondo (2000) Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells.Enzyme Microb. Technol. 27: 43–52.
Glacken, M. W. (1988) Catabolic control of mammalian cell culture.Bio/Technol. 6: 1041–1050.
Lanks, K. W. and P. W. Li (1988) End products of glucose and glutamine metabolism by cultured cell lines.J. Cell Physiol. 135: 151–155.
Petch, D. and M. Butler (1994) Profile of energy metabolism in a murine hybridoma: glucose and glutamine utilization.J. Cell Physiol. 161: 71–76.
Irani, N., M. Wirth, J. van Den Heuvel, and R Wagner (1999) Improvement of the primary metabolism of cell cultures by introducing a new cytoplasmic pyruvate carboxylase reaction.Biotechnol. Bioeng. 66: 238–246.
Ljunggren, J. and L. Haggstrom (1994) Catabolic control of hybridoma cells by glucose and glutamine limited fed batch cultures.Biotechnol. Bioeng. 44: 808–818.
Flickinger, M. C., N. K. Goebel, T. Bibila, and S. Boyce-Jacino (1992) Evidence for posttranscriptional stimulation of monoclonal antibody secretion by L-glutamine during slow hybridoma growth.J. Biotechnol. 22: 201–226.
Leno, M., O. W. Merten, and J. Hache (1992) Kinetic analysis of hybridoma growth and monoclonal antibody production in semicontinuous culture.Biotechnol. Bioeng. 39: 596–606.
Reusch, H. P., J. Lowe, and H. E. Ives (1995) Osmotic activation of a Na+-dependent Cl−/HCO3 − exchanger.Am. J. Physiol. 268: C147-C153.
Freshney, R. I. (1994)Culture of Animal Cells: A Manual of Basic Technique. Wiley-Liss, New York, NY, USA.
Oyaas, K., T. M. Berg, O. Bakke, and D. W. Levine (1989) Hybridoma growth and antibody production under conditions of hyperosmotic stress. pp. 212–220. In: R. E. Spier, J. B. Griffiths, and P. J. Crooy (eds.),Advances in Animal Cell Biology and Technology for Bioprocesses, Butterworth, Kent, UK.
Oyaas, K., T. E. Ellingsen, N. Dyrset, and D. W. Levine (1994) Utilization of osmoprotective compounds by hybridoma cells exposed to hyperosmotic stress.Biotechnol. Bioeng. 43: 77–89.
Kurano, N., C. Leist, F. Messi, S. Kurano, and A. Fiechter (1990) Growth behavior of Chinese hamster ovary cells in a compact loop bioreactor. 1. Effects of physical and chemical environments.J. Biotechnol. 15: 101–111.
Bibila, T. A., C. S. Ranucci, K. Glazomitsky, B. C. Buckland, and J. G. Aunins (1994) Monoclonal antibody process development using medium concentrates.Biotechnol. Prog. 10: 87–96.
Nuss, D. L. and G. Koch (1976) Variation in the relative synthesis of immunoglobulin G and non-immunoglobulin G proteins in cultured MPC-11 cells with changes in the overall rate of polypeptide chain initiation and elongation.J. Mol. Biol. 102: 601–612.
Oh, S. K. W., P. Vig, F. Chua, W. K. Teo, and M. G. S. Yap (1993) Substantial overproduction of antibodies by applying osmotic pressure and sodium butyrate.Biotechnol. Bioeng. 42: 601–610.
Oh, S. K. W., F. K. F. Chua, and A. B. H. Choo (1995) Intracellular responses of productive hybridomas subjected to high osmotic pressure.Biotechnol. Bioeng. 46: 525–535.
Oyaas, K., T. E. Ellingsen, N. Dyrset, and D. W. Levine (1994) Hyperosmotic hybridoma cell cultures: Increased monoclonal antibody production with addition of glycine betaine.BIotechnol. Bioeng. 44: 991–998.
Ozturk, S. S. and B. O. Palsson (1991) Effect of medium osmolarity on hybridoma growth, metabolism, and antibody production.Biotechnol. Bioeng. 37: 989–993.
Park, S. Y. and G. M. Lee (1995) Feasibility study on the use of hyperosmolar medium for improved antibody production of hybridoma cells in a long-term, repeated-fed batch culture.Bioprocess Eng. 13: 79–86.
Reddy, S., K. D. Bauer, and W. M. Miller (1992) Determination of antibody content in live versus dead hybridoma cells: Analysis of antibody production in osmotically stressed cultures.Biotechnol. Bioeng. 40: 947–964.
Reddy, S. and W. M. Miller (1994) Effects of abrupt and gradual osmotic stress on antibody production and content in hybridoma cells that differ in production kinetics.Biotechnol. Prog. 10: 165–173.
Sureshkumar, G. K. and R. Mutharasan (1991) The influence of temperature on a mouse-mouse hybridoma growth and monoclonal antibody production.Biotechnol. Bioeng. 37: 292–295.
Lee, M. S., K. W. Kim, Y. H. Kim, and G. M. Lee (2003) Proteome analysis of antibody-expressing CHO cells in response to hyperosmotic pressure.Biotechnol. Prog. 19: 1734–1741.
Kim, N. S. and G. M. Lee (2002) Response of recombinant Chinese hamster ovary cells to hyperosmotic pressure: effect of Bcl-2 overexpression.J. Biotechnol. 95: 237–248.
Berg, T. M., K. Oyaas, and D. W. Levine (1990) Growth and antibody production of hybridoma cells exposed to hyperosmotic stress. pp. 93–97. In: H. Murakami (ed.).Trends in Animal Cell Culture Technology. VCH, New York, NY, USA.
Lee, G. M. and S. Y. Park (1995) Enhanced specific antibody productivity of hybridomas resulting from hyperosmotic stress is cell line-specific.Biotechnol. Lett. 17: 145–150.
Lilley, D. M., M. F. Jacobs, and M. Houghton (1979) The nature of the interaction of nucleosomes with a eukaryotic RNA polymerase II.Nucleic Acids Res. 7: 377–399.
Walter, W. and V. M. Studitsky (2001) Facilitated transcription through the nucleosome at high ionic strength occurs via a histone octamer transfer mechanism.J. Biol. Chem. 276: 29104–29110.
Ryu, J. S. and G. M. Lee (1997) Effect of hypoosmotic stress on hybridoma cell growth and antibody production.Biotechnol. Bioeng. 55: 565–570.
Huston, J. S., W. W. Fish, K. G. Mann, and C. Tanford (1972) Studies on the subunit molecular weight of beef heart lactate dehydrogenase.Biochemistry 11: 1609–1612.
Dewey, M. M. and J. L. Conklin (1960) Starch gel electrophoresis of lactic dehydrogenase from rat kidneys.Proc. Soc. Exp. Biol. Med. 105: 492–494.
Van der Helm, H. J. (1961) Simple method of demonstrating lactic acid dehydrogenase isoenzyme.Lancet 2: 108.
Starkweather, W. H., H. H. Spencer, E. L. Schwarz, and H. K. Schoch (1966) The electrophoretic separation of lactate dehydrogenase isoenzymes and their evaluation in clinical medicine.J. Lab. Clin. Med. 67: 329–343.
Millar, D. B. S., M. R. Summers, and J. A. Niziolek (1971) Spontaneousin vitro hybridization of LDH homopolymers in the undenatured state.Nat. New. Biol. 230: 117–119.
Blanco, A. and W. H. Zinkham (1963) Lactate dehydrogenase in human testes.Science 139: 601–602.
Voet, D. and J. G. Voet (1995)Biochemistry, pp. 465. John Wiley & Sons, New York, NY, USA.
Brooks, G. A., H. Dubouchaud, M. Brown, J. P. Sicurello, and C. E. Butz (1999) Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle.Proc. Natl. Acad. Sci. USA 96: 1129–1134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, Y.S., Lee, D.Y., Kim, I.Y. et al. Enhancement of erythropoietin production in recombinant Chinese hamster ovary cells by sodium lactate addition. Biotechnol. Bioprocess Eng. 12, 60–72 (2007). https://doi.org/10.1007/BF02931805
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931805