Abstract
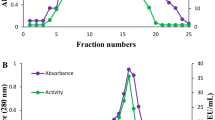
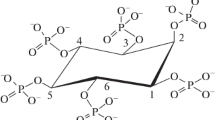
An extracellular acid phosphatase isolated from the culture of a wild strainAspergillus niger, producing the dephosphorylating 3-phytase, was obtained in a homogeneous form by sequential application of ultrafiltration through PS 50 membrane, gel filtration on Sephadex G-100 and ion exchange chromatography on DEAE-Sepharose CL 6B and CM-Sepharose CL 6B. The enzyme showed a maximum catalytic value in a strongly acidic range (pH 2.0–2.4) with pHopt 2.1 andt opt 66 °C. The acid phosphatase showed a wide substrate specificity and a high affinity for sodium phytate, 2.5× higher than with 4-nitrophenyl phosphate. This property of the acid phosphatase demonstrated that it is a potent 3-phytase at pH 2.1 and is of great significance for a practical application of the dephosphorylating complex — its addition to the diets of monogastric animals in view of the low pH values in the digestive tract.
Similar content being viewed by others
References
Dvořáková J.: Phytase sources, preparation and exploitation.Folia Microbiol. 48, 323–338 (1998).
Gargova S., Roshkova Z., Vancheva G.: Screening of fungi for phytase production.Biotech.Techn. 11, 221–224 (1997).
Gargova S., Sariyska M.: Effect of culture conditions on the biosynthesis ofAspergillus niger phytase and acid phosphatase.Enzyme Microb.Technol. 32, 231–235 (2003).
Heinonen J.K., Lahti R.J.: A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase.Anal.Biochem. 113, 313–317 (1981).
Kostrewa D., Wyss M., D’Arcy A., van Loon A.P.G.M.: Crystal structure ofAspergillus niger pH 2.5 acid phosphatase at 2.4 Å resolution.J.Mol.Biol. 288, 965–974 (1999).
Laemmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature227, 680–685 (1970).
Näsi M., Partanen K., Phronen J.: Comparison ofAspergillus niger phytase andTrichoderma reesei phytase and acid phosphatase on phytate phosphorus availability in pigs fed on maize-soybean meal or barley-soybean meal diets.Arch.Anim.Nutr. 52, 15–27 (1999).
Sariyska M., Gargova S., Koleva L., Angelov A.:Aspergillus niger phytase: purification and characterization.Biotechnol.Biotechnol.Eq. 19, 98–106 (2005).
Shimizu M.: Purification and characterization of phytase and acid phosphatase produced byAspergillus oryzae K1.Biosci.Biotech.Biochem. 57, 1364–1365 (1993).
Too K., Kamasaka H., Kusaka K., Kuriki T., Kometani T., Okada S.: A novel acid phosphatase fromAspergillus niger KU-8 that specifically hydrolyzes C-6 phosphate groups of phosphoryl oligosaccharides.Biosci.Biotechnol.Biochem. 61, 1512–1517 (1997).
Ullah A.N.J., Cummins B.J.: Purification, N-terminal amino acid sequence and characterization of pH 2.5 optimum acid phosphatase (EC 3.1.3.2) fromAspergillus ficuum.Prepar.Biochem. 17, 397–422 (1987).
Ullah A.N.J., Gibson D.M.: Extracellular phytase (3.1.3.8) fromAspergillus ficuum NRRL 3135: purification and characterization.Prepar.Biochem. 17, 63–91 (1987).
Ullah A.N.J., Phillippy B.Q.: Substrate selectivity inAspergillus ficuum phytase and acid phosphatases usingmyo-inositol phosphates.Agric.Food Chem. 42, 423–425 (1994).
Ullah A.H.J., Mullaney E.M., Dischinger N.C.: The complete primary structure elucidation ofAspergillus ficuum (niger), pH 6.0, optimum acid phosphatase by Edman degradation.Biochem.Biophys.Res.Comm. 203, 182–189 (1994).
Voříšek J., Kalachová L.: Secretion of acid phosphatase inClaviceps purpurea — an ultracytochemical study.Folia Microbiol. 48, 767–770 (2003).
Wyss M., Pasamontes L., Remy R., Kohler J., Kusznir E., Gadient M., Müller F., van Loon A.P.G.M.: Comparison of the thermostability properties of three acid phosphatases from molds:Aspergillus fumigatus phytase,A. niger phytase andA. niger pH 2.5 acid phosphatase.Appl.Environ.Microbiol. 64, 4446–4451 (1998).
Żyła K.: Acid phosphatases purified from industrial waste mycelium ofAspergillus niger used to produce citric acid.Acta Biotechnol. 10, 319–327 (1990).
Żyła K.: The role of acid phosphatase activity during enzymic dephosphorylation of phytates byAspergillus niger phytase.World J.Microbiol.Biotechnol. 9, 117–119 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gargova, S., Sariyska, M., Angelov, A. et al. Aspergillus niger pH 2.1 optimum acid phosphatase with high affinity for phytate. Folia Microbiol 51, 541–545 (2006). https://doi.org/10.1007/BF02931618
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02931618