Abstract
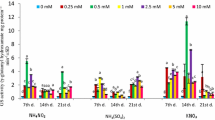
Thein vitro activity of glutamate dehydrogenase (NADH-GDH), from dark-treated root segments of maize seedlings responded differently to amino acids threonine, glutamate and methionine than that from light-treated root segments, and to the amino acid methionine in dark- and light-treated shoot segments. In most cases amino acids inhibited GDH activity, the inhibition increased with amino acid concentration. However, methionine activated GDH from dark-treated roots and light-treated shoots, while aspartate had little effect on enzyme activity.
Similar content being viewed by others
References
Budde, R.J.A., Randall, D.D.: Pea leaf mitochondrial dehydrogenase complex is inactivatedin vivo in a light dependent manner. Proc. nat. Acad. Sci. USA87: 673–676, 1990.
Joy, K.W.: Control of glutamate dehydrogenase fromPisum sativum roots. Phytochemistry12: 1031–1040, 1973.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with Folin phenol reagent. J. biol. Chem.193: 265–275, 1951.
Purnik, R.M., Srivastava, H.S.: Sensitivity to DTNB of NADH-glutamate dehydrogenase from leaves of bean seedlings. Phytochemistry25: 803–805, 1986.
Sahulka, J.: The regulation of nitrate reductase, nitrite reductase and glutamate dehydrogenase in excised pea roots by some exogenous amino acids. Biol. Plant.14: 308–311, 1972.
Singh, R.P., Srivastava, H.S.: Glutamate dehydrogenase activity and assimilation of inorganic nitrogen in maize seedlings. Biochem. Physiol. Pflanz.177: 633–642, 1982.
Singh, R.P., Srivastava, H.S.: Regulation of glutamate dehydrogenase activity by amino acids in maize seedlings. Physiol. Plant.57: 549–554, 1983.
Srivastava, H.S., Singh, R.P.: Role and regulation of L-glutamate dehydrogenase activity in higher plants. Phytochemistry26: 597–610, 1987.
Stewart, G.R., Rhodes, D.: A comparison of the characteristics of glutamine synthetase and glutamate dehydrogenase fromLemna minor. New Phytol.97: 257–268, 1977.
Takeo, T.: Glutamate dehydrogenase from tea(Camellia sinensis) rootlets. Agr. biol. Chem.43: 2257–2264, 1979.
Tischner, R., Aslam, M., Huffaker, R.C.: A cysteine stimulatedin vitro inactivation of barley leaf nitrate reductase. In: Lambers, H., Neeteson, J.J., Stulen, I. (ed.): Fundamental, Ecological and Agricultural Aspects of Nitrogen Metabolism. Pp. 187–191. Martinus Nijhoff Publishers, Dordrecht 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sengar, R.S., Srivastava, H.S. Amino acids response of glutamate dehydrogenase from light and dark treated roots and shoots of maize. Biol Plant 34, 149–152 (1992). https://doi.org/10.1007/BF02925811
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02925811