Abstract
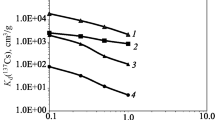
In order to confirm the similar behavior of Eu and Am in heterogeneous geological materials, we carried out the batch experiments for determining the sorption property of radionuclides,152Eu and241Am. We used four different types of core rocks including biotite banded gneiss, biotite gneiss, metabasite and andestic tuff, and selected two samples per each lithology, one of which is fracture-bearing and another is fracture-free. Except for metabasites, rock samples of each type are similar in their compositions. We calculated sorption ratios of two radionuclides from the experimental results. Biotite gneiss and tuff had similar sorption trends for152Eu and241Am regardless of the existence of fractures, whereas two metabasite samples showed very different sorption properties. Such difference in the sorption trends revealed a close relationship with chemical compositions of the host rocks. Nevertheless,152Eu and241Am showed similar adsorption trends for all the samples with variable contact times regardless of petrography and pH variations, and particularly, the sorption trends of152Eu and241Am in the metabasites were similar. This observation suggests that Eu and Am have similar sorption properties on geological materials. Therefore, Eu can be used as a useful analogue of Am in all kinds of geological environments regardless of variations in lithology and pH of groundwater. In addition, sorption ratios of152Eu and241 Am are correlated with the contents of P2O5 and TiO2, suggesting that the chemical components such as P2O5 and TiO2 might be important for deciphering the interaction between the radionuclide and groundwater.
Similar content being viewed by others
References
Berry, J.A. and Bond, K.A., 1992, Studies of the Extent of Surface Diffusion in the Migration of Radionuclides through Geological Materials. Radiochimica Acta, 58/59, 329–335.
Buddemeier, R.W., Finkel, R.C., Marsh, K.V., Ruggieri, M.R., Rego, J.H. and Silva, R.J., 1991, Hydrology and Radionuclide at the Nevada Test Site. Radiochimica Acta, 52/53, 275–282.
Coppin, G.R., 1983, Comparison of the solution chemistry of the actinides and the lanthanides. Journal of Less-Common Metals, 93, 232–230.
Curti, E., 1999, Coprecipitation of radionuclides with calcite: estimation of partition coefficients based on a review of laboratory investigations and geochemical data. Applied Geochemistry, 14, 433–445.
Degueldre, C. and Wernli, B., 1993, Association Behavior of241Am(III) on SiO2(amorphous) and SiO2(quartz) Colloids. Journal of Environmental Radioactivity, 20, 151–167.
Derry, L.A. and Jacobsen, S.B., 1990, The chemical evolution of Precambrian scawater: Evidence from REEs in banded iron formations. Geochimica Cosmochimica Acta, 49, 1955–1963.
Dymek, R.F. and Klein, C., 1988, Chemistry, petrography and origin of banded iron-formation lithologies from the 3800 Ma Isua supracrustal rocks. West Greenland, Precambrian Research, 39, 247–302.
Emrén, A.T., 1993, The influence of heterogeneous rock chemistry on the sorption of radionuclides in flowing groundwater. Journal of Contaminant Hydrology, 13, 131–141.
Fred, M., 1967, in Lanthanide/Acitinide Chemistry, vol. 71, Advances in Chemistry Scries, American Chemical Society, Washington, pp. 200–201.
Guiterrez, M.G., Bidoglio, G., Avogadro, A., Mingarro, E. and D'Alessandro, M., 1991, Experimental Investigations of radionuclide transport through cored granite samples. Radiochimica Acta, 52/53, 213–217.
Heath, M.J., Montoto, M., Rodriguez Rey, A., Ruiz de Argandoña, V.G. and Menendez, B., 1992, Rock Matrix Diffusion as a Mechanism of Radionuclide Retardation: A Natural Analogue Study of El Berrocal Granite, Spain. Radiochimica Acta, 58/59, 379–384.
Henderson, P., 1990, Inorganic Geochemistry. Pergamon Press, Oxford-New York, 353 p.
Hilton, J., Nolan, L. and Jarvis, K.E., 1997, Concentrations of stable isotopes of cesium and strontium in freshwaters in northern England and their efforts on estimates of sorption coefficients (Kd). Geochimica Cosmochimica Acta, 61, 1115–1124.
Ionova, G., Madic, C. and Guillaumont, R., 1997, Covalency Effects in the Standard Enthalpies of Formation of Trivalent Lanthanide and Actinide Halides. Radiochimica Acta, 78, 83–90.
Johannesson, K., Stetzenbach, K.J., Hodge, V.H. and Lyons, W.B., 1996, Rare earth element complexation behaviour in circumncutral pH groundwaters: Assessing the role of carbonate and phosphate ions. Earth and Planetary Science Letters, 139, 305–319.
Johansson, A. and Rosengren, A., 1975, Interpolation scheme for the cohesive energies for the lanthanides and actinides. Physical Review, B11, 1367–1373.
Kim, M.A., Panak, P.J., Yun, J.I., Kim, J.I., Klenze, R. and Köhler, K., 2003, Interaction of actinides with aluminosilicate colloids in statunasscendi. Part I; generation and characterization on actinide (III)-pseudocolloids. Colloidal Surface, A 216, 97–108.
Konta, J., 1995, Clay and man: Clay raw materials in the service of man. Applied Clay Science, 10, 275–335.
Krauskopf, K.B., 1986a, Aqueous geochemistry of radioactive waste disposal. Applied Geochemistry, 1, 15–23.
Krauskopf, K.B., 1986b, Thorium and rare carth metals as analogs for actinide elements. Chemical Geology, 55, 323–335.
Lee, K.Y., Yoon, Y.Y., Lee, S.G., Lee, D.H., Kim, Y. and Woo, N.C., 2001, Sorption of radionuclides on the container wall during batch migration studies. Journal of Radioanalytical Nuclear Chemistry, 249, 271–278.
Lee, S.G., Lee, D.H., Kim, Y., Chac, B.G., Kim, W.Y. and Woo, N.C., 2003, Rare carth elements as an indicator of groundwater environment changes in a fractured rock system: Evidence from fracture-filling calcite. Applied Geochemistry, 18, 135–143.
Lee, S.G., Kim, Y., Chac, B.G., Koh, D.C. and Kim, K.H., 2004, The geochemical implication of a variable Eu anomaly in a fractured gneiss core: application for understanding Am behavior in the geological environment. Applied Geochemistry, 19, 1711–1725.
Lipin, B.R. and McKay, G.A., 1989, Geochemistry and Mineralogy of Rare Earth Elements. Reviews in Mincralogy, vol. 21, Mineralogical Society of America, 348pp.
Masuda, A., Nakamura, N. and Tanaka, T., 1973, Finc Structure of mutually normalized rare-earth patterns of chondrites. Geochimica Cosmochimica Acta, 37 239–248.
McCarthy, J.F., Sanford, W.E. and Stafford, P.L., 1998, Lanthanide Field Tracers Demonstrate Enhanced Transport of Transuranic Radionuclides by Natural Organic Matter. Environmental Science and Technology, 32, 3901–3906.
Meece, D.E. and Benninger, L.K., 1993, The coprecipitation of Pu and other radionuclide with CaCO3 Geochimica Cosmochimica Acta, 57, 1447–1458.
Metz, V., Kienzler, B. and Schûßler, W., 2003 Geochemical evaluation of different groundwater-host rock systems for radioactive waste disposal. Journal of Contaminant Hydrology, 61, 265–279.
Moeller, T., 1976, The Lanthanides. In: Comprehensive Inorganic Chemistry (J.C. Bailar et al. ed), 1–101, Pergamon Press, Oxford-New York-Toronto-Sydney-Braunschweig.
Nagasaki, S., Tanaka, S. and Suzuki, A., 1997, Interfacial behavior of actinides with colloids in the geosphere. Journal of Nuclear Materials, 248 323–327.
O'Connor, J.T., 1965, A classification for quartz-rich igneous rocks based on feldspar ratio. United States Geological Survey Professional Paper, 525B, 79–84.
Petit J-C., 1991, Natural Analogue Aspects of Radionuclide Transport in the Geosphere. Radiochimica Acta, 52/53, 337–340.
Rai, D., Strickert, R.G., Moore, D.A. and Serne, R.J., 1981, Influence of an americium solid phase on americium concentrations in solutions. Geochimica Cosmochimica Acta, 45, 2257–2265.
Rizkalla, E.N. and Choppin, G.R., 1991, Chap. 103 in Handbook on the Physics and chemistry of Rare Earths, Vol. 15, Gshncidner, K. A. Jr. and Eyring, L. Eds., Elsevier, New York.
Runde, W., Meinrath, G. and Kim, J.I., 1992, A Study of Solid-Liquid Phase Equilibria of Trivalent Lanthanide and Actnide Ions in Carbonate Systems. Radiochimica Acta, 58/59, 93–100.
Sakuragi, T., Sato, S., Kozaki, T., Mitsugashira, T., Hara, M. and Suzuki, Y., 2004, Am(III) and Eu(III) uptake on hematite in the presence of humic acid. Radiochimica Acta, 92, 697–702.
Shannon, R.D., 1976, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalkogenides. Acta Crystallogrography, 32, 751–767.
Spasennykh, M.Yu. (1997) Radionuclide Migration in Aquifer Rocks: Effect of Water-Rock Interaction. Geochemistry International, 35, 181–187.
Stipp, S.L.S., Lakstanov, L.Z., Jensen, J.T. and Baker, J.A., 2003, Preliminary results from coprecipitation experiments and observations with surface-sensitive techniques. Journal of Contaminant Hydrology, 61, 33–43.
Takahashi, Y., Kimura, T., Kato, Y., Minai, Y. and Tominaga, T. 1998, Characterization of Eu(III) Species Sorbed on Silica and Montmorillonite by Laser-Induced Fluorescence Spectroscopy. Radiochimica Acta, 82, 227–232.
Takahashi, Y., Yoshida, H., Sato, N., Hama, K., Yusa, Y. and Shimizu, H., 2002, W- and M-type tetrad effects in REE patterns for waterrock systems in the Tono uranium deposits, central Japan. Chemical Geology, 184, 311–335.
Taylor, S.R. and McLennan, S.M., 1985, The continental Crust: Its Composition and Evolution. Blackwell. 312p.
Wood, S.A., 1990, The aqueous geochemistry of the rare earth elements and Yttrium. I. Review of the available low-temperature data for inorganic complexes and inorganic REE speciation in natural waters. Chemical Geology, 82, 159–186.
Xu, S. and Wörman, A., 1999, Implications of sorption kinetics to radionuclide migration in fractured rock. Water Resource Research, 35, 3429–3340.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SG., Lee, K.Y., Cho, S.Y. et al. Sorption properties of152Eu and241 Am in geological materials: Eu as an analogue for monitoring the Am behaviour in heterogeneous geological environments. Geosci J 10, 103–114 (2006). https://doi.org/10.1007/BF02910354
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02910354