Abstract
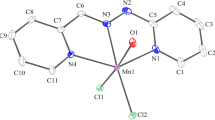
The crystal structure of the mono- and heterometallic complexes [MEn3]L2 (M = Zn2+,M2+) and [MEn3 ][CdL3]2 (M = Cd2+, Zn2+, Ni2+) containing ethylenediamine (En) molecules and diethyldithio-carbamate ions (L = (C2H5)2NCS −2 ) is investigated. The three heterometallic complexes are isostructural; their structure consists of discrete mononuclear ions. In the [MEn3]2+ cation, the central M atom lies on the twofold axis; therefore, two of the three metallocycles MN2C2 of the [MEn3]2+ cation are independent. One of the two has a gosh-configuration. In the coordinated En molecules, the N-M-N chelate angles are 77.0 and 82.9‡ (M = Cd2+); 80.0 and 80.5‡ (M = Zn2+); 79.7 and 80.8‡ (M = Ni2+). The nitrogen atoms form a distorted octahedron around M. The average M-N bond lengths for the complexes are 2.35, 2.19, and 2.16 å for M = Cd2+, Zn2+, and Ni2+, respectively. All the atoms of the [CdL3]− complex anion are in the general position; the central atom coordinates three cyclic bidentate L− ligands. The S atoms form a distorted trigonal prism, where the S...S distances in the vertical edges are nearly the same in all the complexes (2.94(1)-3.00(2) å). It was shown by1H,14N, and113Cd NMR that the ionic complexes [ZnEn3]L2 and [CdEn3][CdL3]2 in solution are transformed into nonelectrolyte type mixed-ligand complexes.
Similar content being viewed by others
References
D. Coucouvanis,Prog. Inorg. Chem.,11, 234–371 (1970).
D. Coucouvanis,ibid.,26, 301–469 (1979).
S. V. Larionov,Zh. Neorg. Khim.,24, 1446–1456 (1979).
S. V. Larionov,ibid.,38, 1616–1624 (1993).
J. O. Hill and R. J. Magee,Rev. Inorg. Chem.,3, 141–197 (1981).
V. M. Byrko,Dithiocarbamates [in Russian], Nauka, Moscow (1984).
S. V. Larionov, V. N. Kirichenko, S. M. Zemskova, and I. M. Oglezneva,Koordinats. Khim.,16, 79–84 (1990).
S. M. Zemskova, S. A. Gromilov, and S. V. Larionov,Sib. Khim. Zh., No. 4, 74–77 (1991).
L. A. Glinskaya, R. F. Klevtsova, and S. M. Zemskova,Zh. Strukt. Khim.,33, No. 1, 106–114 (1992).
S. M. Zemskova, L. A. Glinskaya, R. F. Klevtsova, et al.,ibid.,34, No. 5, 157–166 (1993).
S. M. Zemskova, L. A. Glinskaya, R. F. Klevtsova, and S. V. Larionov,Zh. Neorg. Khim.,38, No. 3, 466–471 (1993).
S. M. Zemskova, L. A. Glinskaya, R. F. Klevtsova, et al.,Zh. Strukt. Khim.,36, No. 3, 528–540 (1995).
B. F. Abrahams, B. F. Hoskins, and G. Winter,Aust. J. Chem.,43, 1759–1765 (1990).
L. A. Glinskaya, S. M. Zemskova, R. F. Klevtsova, et al.,Polyhedron, No. 22, 2951–2956 (1992).
L. A. Glinskaya, S. M. Zemskova, and R. F. Klevtsova,Zh. Strukt. Khim.,39, No. 2, 353–359 (1998).
F. Alien, S. Bellard, U. D. Brice, et al.,Acta Crystallogr.,B35, 2331–2337 (1979).
S. M. Zemskova, “Mixed-ligand and heterometallic complex compounds of zinc(II), cadmium(II), and mercury(II) containing dialkyldithiocarbamate anions,” Chemical Sciences Candidate’s Dissertation, Novosibirsk (1994).
T. N. Fedotova, M. A. Fedotov, and I. F. Golovaneva,Zh. Neorg. Khim.,40, No. 8, 1355–1362 (1995).
Author information
Authors and Affiliations
Additional information
Translated fromZhurnal Strukturnoi Khimii, Vol. 40, No. 2, pp. 340–350, March–April, 1999.
Rights and permissions
About this article
Cite this article
Zemskova, S.M., Glinskaya, L.A., Klevtsova, R.F. et al. Structure and properties of mono- and heterometallic cadmium, zinc, and nickel complexes containing diethyldithiocarbamate ions and ethylenediamine molecules. J Struct Chem 40, 284–292 (1999). https://doi.org/10.1007/BF02903658
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02903658