Abstract
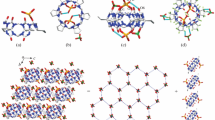
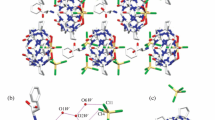
Self-assembled complexes between cage compounds cucurbit[n = 5–8]urils and hexamethylenetetramine were studied by using NMR techniques. Experimental results reveal that hexamethylenetetramine can lid cucurbit [5] uril to forming self-assembled capsules in which nothing is encapsulated yet; the cavity of the cucurbit[7]uril can accommodate a hexamethylenetetramine molecule to form a selfassembled host-guest inclusion. Moreover, both the cavity interaction of the cucurbit[7]uril with hexamethylenetetramine·HCl and the portal interaction of the dipole carbonyl of the cucurbit[7]uril with hexamethylenetetramine·HCl lead to form self-assembled capsules in which the hexamethylenetetramine·HCl are encapsulated in the hexamethylenetetramine·HCl “lidded” cucurbit[7]uril. Although the structures of the portal and cavity to cucurbit[5]uril are similar, there is no obvious interaction between decamethylcucurbit[5]uril and hexamethylenetetramine, and also between cucurbit [6]uril or cucurbit[8]uril and hexamethylenetetramine.
Similar content being viewed by others
References
Liu, Y., You, C. C., Zhang, H. Y., Supramolecular Chemistry (in Chinese), Tianjin: Nankai University Press, 2001, 385–448.
Winston, O., Marielle, G. K., Angel, E. et al., New-Cucurbit[7]uril: A Very Effective Host for Viologens and Their Cation Radicals, Organic Letters, 2002, 4(10): 1791–1794
Jansen, K., Buschmann, H. J., Zliobaite, E. et al., New-Steric factors influencing the complex formation with cucurbit[6]uril, Thermochimica Acta, 2002, 385(1-2): 177–184.
Freeman, W. A., Mock, W. L., Shih, N. Y., Cucurbituril, J. Am. Chem. Soc., 1981, 103: 7367–7368.
Day, A. I., Arnold, A. P., Method for synthesis cucurbiturils, WO 0068232, 2000, 8.
Kim, J., Jung, I. S., Kim, S. Y. et al., New cucurbituril homologues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8), J. Am. Chem. Soc., 2000, 122(3): 540–541.
Day, A. I., Blanck, R. J., Arnold, A. P., A cucurbituril-based gyroscane: a new supramolecular form, Angew Chem. Int. Ed, 2002, 41(2): 275–277.
Neugebauer, R., Knoche, W., Host-guest complexes of cucurbituril with 4-amino-4-nitroazobenzene and 4,4′-diaminoazobenzene in acidic aqueous solutions, J. Chem. Soc., Perkin Trans. 2, 1998, 3: 529–534.
Jeon, Y. M., Heo, J., Whang, D. et al., Molecular container assembly capable of controlling binding and release of its guest molecules: reversible encapsulation of organic molecules in sodium ion complexed cucurbituril, J. Am. Chem. Soc, 1996, 118(40): 9790–9791.
Whang, D., Heo, J., Park, J. H. et al., A molecular bowl with metal ion as bottom: reversible inclusion of organic molecules in cesium ion complexed cucurbituril, Angew. Chem. Int. Ed. Engl., 1998, 37(1/2): 78–80.
Blanck, R., Sleeman, Day, A. I., Cucurbit[7]uril and o-carborane self-assemble to form a molecular ball bearing, Nano Lett., 2002, 2(2): 147–149.
Kim, S. Y., Jung, I. S., Kim, K., Macrocycles within macrocycles: cyclen, cyclam, and their transition metal complexes encapsulated in cucurbit[8]uril, Angew Chem. Int. Ed. Engl., 2001, 40(11): 2119–2121.
Yao, X. Q., Shen, Y. Q., Tao, Z. et al., New cage compounds–Cucurbit[n]uril (n = 5,7 and 8,), Journal of Guizhou University (in Chinese), 2003, 20(1): 105–110.
Han, B. H., Liu, Y., Molecular Recognition and Assembly of cucurbituril, Chinese Journal of Inorganic Chemistry, 2003, 23(2): 139–149.
Lorenzo, S., Day, A. I., Craig, D. et al., The first endoannular metal halide-cucurbituril: cis-SnCl4(OH2)2@cucurbit[7]uril, Cryst. Eng. Comm., 2001, 49: 1–7.
Samsonenko, D. G., Virovets, A. V., Lipkowski, J. et al., Newdistortion of the cucurbituril molecule by an Included 4-methylpyridinum cation. Journal of Structural Chemistry (Translation of Zhurnal Strukturnoi Khimii), 2002, 43(4): 664–668.
Samsonenko, D. G., Gerasko, O. A., Lipkowski, J. et al., New synthesis and crystal structure of the nanosized supramolecular SmIII complex with macrocyclic cavitand cucurbituril [Sm(H2O)4]2(C36H36N24O12)3Br6 · 44H2O, Russian Chemical Bulletin (Translation of Izvestiya Akademii Nauk, Seriya Khimicheskaya), 2002, 51(10): 1915–1918.
Zhang, G. L., Xu, Z. Q., Xue, S. F. et al., A New Family of Cage Compounds Cucurbit[n]urils: (II) Influence of Acidity, Alkaline and Alkaline-earth Metal Ions on Solubility of Cucurbit[n = 5—8]urils, Chinese Journal of Inorganic Chemistry (in Chinese), 2003, 19(6): 655–659.
Flinn, A., Hough, G. C., Stoddart, J. F. et al., Decamethylcucurbit[5]uril, Angew. Chem., Int. Ed. Engl., 1992, 31(11): 1475–1477.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shen, Y., Xue, S., Zhao, Y. et al. NMR study on self-assembled cage complex of hexamethylenetetramine and cucurbit[n]urils. Chin. Sci. Bull. 48, 2694–2697 (2003). https://doi.org/10.1007/BF02901758
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02901758