Abstract
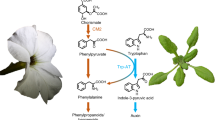
In pea, maize and tomato plants a hitherto undescribed L-tryptophan dehydrogenase activity (TDH) has been detected. This enzyme catalyzes the reversible formation of indolepyruvic acid (IPyA) from L-tryptophan (L-trp). TDH and L-glutamate dehydrogenase (GDH), related enzymes in their mode of action, could be separated by gel chromatography. Enzymatic activity of TDH was sustained by both pyridine coenzymes NAD/NADP. With pea TDH the coenzyme NAD displays, at optimum pH 8.5 and at room temperature, only about 40-70 % of the activity of NADP. The amination of IPyA is catalysed more actively than the deamination of L-trp. L-trp/IPyA, L-glu/ketoglutarate, L-ala/pyruvate reacted as dehydrogenase substrates; L-phe/ phenylpyruvate, D-trp and D-phe did not react with pea enzyme extracts. A considerable similarity between the active centres of TDH and GDH has been found using inhibitors: absence of heavy metals, presence of a carbonyl group, indispensibility of bivalent ions for the enzyme activity. Pea TDH and GDH were distinctly inhibited by sodium azide. For the activity of TDH the presence of SH groups is less important than for GDH. The TDH activity in the investigated plants was lower than the GDH activity. The possible role of TDH in the regulation of the IPyA pool is discussed.Doc. RNDr. PhMr. M. Kutáček died on 28 November, 1989. The final form for print was prepared by dr. Ivana Machdckovd of the same Institute, who will also answer the reprint requests. Received June 6, 1990; accepted October 10, 1990
Similar content being viewed by others
References
Bradford, M. M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. -Anal. Biochem.72: 248–254, 1976.
Davies, D. D., Giovanelli, J., Ap Rees, T.: Plant Biochemistry. -F. A. Davies Co., Philadelphia 1964.
Ebeid, M. M., Dimova, S. D., Kutáček, M.: Substrate specificity of L-tryptophan dehydrogenase and its distribution in plants. -Biol. Plant.27: 413–416, 1985.
Heilmann, B., Hartung, W., Gimmler, H.: Subcellular compartmentation of indole-3-acetic acid in mesophyll cell ofSpinacea oleracea. -Z. Naturforsch. C36: 697–685, 1981a.
Heilmann, B., Hartung, W., Gimmler, H.: The site of indole-3-acetic acid synthesis in mesophyll cells ofSpinacea oleracea. -Z. Naturforsch.C 37: 174–178, 1981b.
Krause, J., Bühner, M., Sund, H.: Studies of glutamate dehydrogenase. The binding of NADH and NADPH to beef-liver glutamate dehydrogenase. -Eur. J. Biochem.41: 593–602, 1974.
Kutáček, M.: Auxin biosynthesis and its regulation on the molecular level. -Biol. Plant.27:145–153, 1985.
Kutáček, M., Kefeli, V. I.: The present knowledge of indole compounds in plants of theBrassicaceae family. -In: Wightman, F., Setterfield, G. (ed.): Biochemistry and Physiology of Plant Growth Substances. Pp. 127–152. The Runge Press, Ottawa 1968.
Kutáček, M., Kralova, M.: Biogenesis of the glucobrassicin aglycon from14C and15N labelled L-tr.yptophan precursors. -Biol. Plant.14: 279–285, 1972.
Mahadevan, S., Stowe, B.: Conversion of 3-indoleacetaldoxime to glucobrassicin and sulphog-lucobrassicin by woad(Isatis tinctoria L.). -In: Carr, D.(ed.): Plant Substances 1970. Pp 117–126. Springer Verlag, Heidelberg 1972.
Muir, R. M., Lantican, B. P.: Purification and properties of the enzyme system forming indoleacetic acid. -In: Wightman, F., Setterfield, G.(ed.): Biochemistry and Physiology of Plant Growth Substances. Pp. 259–272. The Runge Press, Ottawa 1968.
Sahulka, J.: The effect of exogenous IAA and kinetin on nitrate reductase and glutamate dehydrogenase activities in excised pea roots.-Biol. Plant.14: 330–336, 1972.
Sandberg, G., Jensen, E., Crozier, A.: Biosynthesis of indole-3-acetic acid in protoplasts, chloroplasts and cytoplasmic fraction from barley(Hordeum vulgare L.). -Planta156: 541–543, 1982.
Schneider, E. A., Wightman, F.: Auxins. -In: Letham, D. S., Goodwin, R. H., Higgins, R. H. (ed.): Phytohormones and Related Compounds. Pp. 29–105. Elsevier/North-Holland Biomedical Press, Amsterdam 1978.
Suzuki, Y., Kamisaka, S., Yanagisawa, H., Miyata, S., Masuda, Y.: Effect of light on growth and metabolic activities in pea seedlings II. Changes in the IAA content and activities of enzymes involved in the IAA biosynthesis during growth. -Biochem. Physiol. Pflanzen176: 35–43, 1981.
Terziivanova-Dimova, S. D., Kutáček, M.: Enzymes of auxin biosynthesis and their regulation I. Tryptophan and phenylalanine aminotransferase. -Biol. Plant. In press.
Truelsen, T. A.: Indole-3-pyruvic acid as an intermediate in the conversion of tryptophan to indole-3-acetic acid. I. Some characterizations of tryptophan transaminase from mung bean seedling. -Physiol. Plant.26: 282–295, 1972.
Vackova, K., Mehta, A., Kutáček, M.: Tryptophan aminotransferase and tryptophan dehydrogenase activities in some cell compartments of spinach leaves: the effect of calcium ions on tryptophan dehydrogenase. -Biol. Plant.27: 154–158, 1985.
Wightman, F., Cohen, N. D.: Intermediate steps in the enzymatic conversion of tryptophan to IAA in cell. -In: Wightman, F., Setterfield, D. (ed.): Biochemistry and Physiology of Plant Growth Substances. Pp. 273–288. The Runge Press, Ottawa 1968.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kutaček, M., Terziivanova-dimova, S. Proposed Enzymes of Auxin Biosynthesis and Their Regulation II. Tryptophan Dehydrogenase Activity in Plants.. Biol Plant 33, 395–407 (1991). https://doi.org/10.1007/BF02897691
Issue Date:
DOI: https://doi.org/10.1007/BF02897691