Abstract
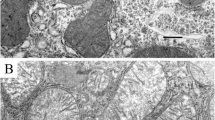
The localization of the formic dehydrogenase system was studied by means of nitro-blue tetrazolium, using the ultrathin section technique, in large long bodies ofProteus vulgaris, which form the intermediate stage in regeneration of penicillin-induced spheroplasts. In the electron microscope, empty areas, numbering on an average four per cell section, were found at the site of formazan deposits, while in the controls (without tetrazolium salts) the average number was 0.05. From these findings, and from the findings of other authors showing that formic dehydrogenase is bound to cell structures, it can be concluded that inProteus cells this enzyme is bound to the cytoplasmic membrane.
Abstract
Локализация системы дегидрогеназы муравьиной кислоты исследовалась с применением nitro-blue-тетразолияитехники ультратонких срезов. Объектом исследования являлись крупные продолговатые тельца Proteus vulgaris, представляющие собой переходную стадию в процессе регенерации пенициллиновых сферопластов. В месте расположения формазана под электронным микроскопом были обнаружены пустоты, средним числом по 4 на 1 срез через клетку, тогда как в контроле (без соли тетразолия) их приходилось только 0,05 на 1 срез. На основании зтих наблюдений, а также данных других авторов, что дегидрогеназа муравьиной кислоты связывается с клеточными структурами, можно полагать, что в клетках протея этот фермент связан с цитоплазматической оболочкой.
Similar content being viewed by others
References
Beneš, K., Lojda, Z., Hořavka, B.:Contribution to the histochemical demonstration of some hydrolytic and oxidative enzymes in plants. Histochemie 2: 313, 1961.
Davidson, D. C.:Studies on plant formic dehydrogenase. Biochem. J. 49:520, 1951.
Feldman, W., O'Kane, D. J.:Some additional properties of Proteus particles. J. Bacteriol. 80:218, 1960/2.
Fitz-James, P. C.:Participation of the cytoplasmatic membrane in the growth and spore formation of Bacilli. J. Biophys. Biochem. Cytol. 8:507, 1960.
Giesbrecht, P.:Über “organisierte” Mitochondrien und andere Feinstrukturen von Bacillus megaterium. Zbl. Bakt. I Orig. 179:538, 1960.
Glauert, A. M., Hopwood, D. A.:The fine structure of Streptomyces coelicolor. I. The cytoplasmatic membrane system. J. Biophys. Biochem. Cytol. 7: 479, 1960.
Iterson, W. van:Membranes, particular organelles and peripheral bodies in bacteria. Proc. Eur. Reg. Conf. on Electron Microscopy, Vol. II. p. 763, Delft 1960.
Iterson, W. van:Some features of a remarkable organelle in Bacillus subtilis. J. Biophys. Biochem. Cytol. 9:183, 1961.
Nachlas, M. M., Tsou, K. C., de Souza, E., Cheng, C. S., Seligman, A. M.:Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J. Histochem. Cytochem. 5:420, 1957.
Nachlas, M. M., Walker, D. G., Seligman, A. M.:The histochemical localization of triphosphopyridine nucleotide diaphorase. J. Biophys. Biochem. Cytol. 4:467, 1958.
Nermut, M.:L-forms of bacteria. IV. The influence of temperature on the development of the L-cycle of Proteus vulgaris and its significance for the development of resistance to penicillin. Fol. biol. (Praha) 3:149, 1957.
Nermut, M.:L-cyklus bakterií, jeho biologický a lékařský význam. Státní zdravotnické nakl., Praha 1960.
Niklowitz, W.:Mitochondrienäquivalente bei Escherichia coli. Zbl. Bakt. I Origin. 173:12, 1958.
Nossal, P. M., Keech, D. B., Moton, Dr. L.:Respiratory granules in microorganisms. Biochim. Biophys. Acta 22:412, 1956.
Novikoff, A. B., Shin, W., Drucker, J.:Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J. Biophys. Biochem. Cytol. 9:47, 1961.
Palade, G. E.: J. Exp. Med. 95:285, 1952. Cited by Pease, 1960.
Pearse, A. G. E.:Intracellular localization of dehydrogenase systems using monotetrazolium salts and metal chelation of their formazans. J. Histochem. Cytochem. 5:515, 1957.
Pease, D. C.:Histological techniques for electron microscopy. Academic Press, New York and London 1960.
Peck, H. D., Gest, H.:Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J. Bacteriol. 73:706, 1957.
Sedar, A. W., Rosa, C. G.:Cytochemical demonstration of the succinic dehydrogenase system with the electron microscope using Nitro-blue tetrazolium. J. Ultrastructure Res. 5:226, 1961.
Schulze, W., Butschak, G.:Der Elektronenmikroskopische Nachweis von Dehydrogenasen im Herzmuskel verschiedener Versuchstiere. Acta histochem. 14:260, 1962.
Ševčík, V.:Úvod do biochemické analysy mikrorganismů. Nakl. Čsl. akademie věd, Praha 1954.
Tsou, K. C., Cheng, C. S., Nachlas, M. M., Seligman, A. M.:Syntheses of some p-nitrophenyl substituted tetrazolium salts as electron acceptors for the demonstration of dehydrogenase. J. Amer. Chem. Soc. 78:6139, 1956.
Wrigley, C. W., Linnane, A. W.:Formic dehydrogenase-cytochrome B 1 complex from Escherichia coli. Biochem. Biophys. Res. Commun. 4:66, 1961.
Yaeger, J. A.:Microscopic and submicroscopic localization of succinic dehydrogenase activity in the muscle cells of mouse diaphragm. Exp. Cell Res. 22:439, 1961.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nermut, M.V., Rýc, M. Electron microscopy of the localization of formic dehydrogenase in regenerating forms ofProteus vulgaris . Folia Microbiol 9, 16–20 (1964). https://doi.org/10.1007/BF02875895
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02875895