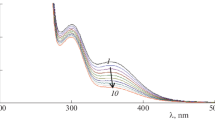
Abstract
Reactions of equimolar mixtures of copper (Cu), zinc (Zn) metal powders with either ammonium formate or acetate or tetrabutyl ammonium acetate (1:1:2) in excess hydrogen peroxide (H2O2) at ambient conditions results in decarboxylation yielding mixed metal oxide [Cu, ZnO2·H2O], while the corresponding reaction with ammonium succinate results in formation of mixed metal peroxo carbonate [Cu, Zn(O 2−2 )(CO3)(H2O)2]. Interestingly, in all the above mentioned carboxylates, the corresponding reactions in the presence of H2O2 and imidazole (HIm) results in formation of HIm adducts of mixed metal peroxo carbonates [Cu, Zn(O 2−2 )(CO3)(HIm)x(H2O)2]. It is noteworthy to mention that all the above mentioned reactions in presence of either Cu or Zn powder alone, did not give any homogeneous reaction product, and instead the metal powder remained undissolved. Similarly, it is very significant to observe that the reaction of sodium salts of the above mentioned carboxylic acids with H2O2 in presence of either Cu-Zn mixture or Cu-Zn-HIm mixture did not give any decomposed products and instead the metal powders remained undissolved. Hence this work is of significance as it reports for the first time, the profound effect of the presence of both ammonium ion and carboxylic group in simple carboxylates and its reaction with H2O2 in the presence of Cu-Zn mixture. The new mixed metal peroxo carbonates and the imidazole adducts isolated from the above mentioned reaction systems were characterised by chemical, elemental and thermogravimetric analyses, ESR, electronic and IR spectral studies.
Similar content being viewed by others
References
Sastry M S, Gupta S S, Natarajan V and Singh A J 1992J. Inorg. Biochem. 45 159
Sastry M S, Gupta S S and Singh A J 1994J. Coord. Chem. 31 353
Sastry M S, Gupta S S and Singh A J 1995Biometals 8 174
Nakamoto K, Fujita J, Tanaka S and Kotayashi M 1957J. Am. Chem. Soc. 79 4904
Gixue, Jiang S, Huangx and Gaoguanshi 1988J. Chem. Soc., Dalton Trans. 1487
Stevansad S, Helland B J, Babich M W, Jacobson R A and McCarley 1978J. Am. Chem. Soc. 100 6258
Kitajima N, Koda T, Hashimoto S, Kitagava T and Moro-Okay 1991J. Am. Chem. Soc. 113 5664
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sastry, M.S., Gupta, S.S. Effect of some transition metals on interactions of simple ammonium carboxylates with hydrogen peroxide. Proc. Indian Acad. Sci. (Chem. Sci.) 110, 415–419 (1998). https://doi.org/10.1007/BF02872574
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02872574