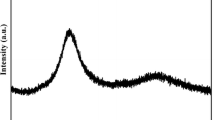
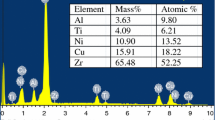
Abstract
Differential scanning calorimeter (DSC) is used to investigate apparent activation energy of glass transition and crystallization of Zr-based bulk amorphous alloys by Kissinger equation under non-isothermal condition. It is shown that the glass transition behavior as well as crystallization reaction depends on the heating rate and has a characteristic of kinetic effects. After being isothermally annealed near glass transition temperature, the apparent activation energy of glass transition increases and the apparent activation energy of crystallization reaction decreases. However, the kinetic effects are independent of the pre-annealing.
Similar content being viewed by others
References
Peker, A., Johnson, W. L., A highly processably metallic glass: Zr41.2Ti13.8Cu12.5Ni10.0Be22.5, Appl. Phys. Lett., 1993, 63: 2342.
Wang Weihua, Wang Wenkui, Bai Hai Yang, Formation of New Zr-Ti-Cu-Ni-Be-C bulk amorphous alloys, Science in China (in Chinese), Ser. A, 1998, 28: 443.
Inoue, A., Zhang, T., Nishiyama, N. et al., Preparation of 16 mm diameter rod of amorphous Zr65Al7.5Ni10Cu17.5 alloy, Mater. Trans. JIM, 1993, 34: 1234.
Shen, T. D., Schwarz, R. B., Bulk ferromagnetic glasses prepared by flux melting and water quenching, Appl. Phys. Lett., 1999, 75: 49.
Kelton, K. F., A new model for nucleation in bulk metallic glasses, Phil. Mag. Lett., 1998, 77: 337.
Tsao, S. S., Spaepen, F., Structural relaxation of a metallic glass near equilibrium, Acta Metall., 1989, 33: 881.
Wang, W. H., Wei, Q., Friedrich, S., Microstructure, decomposition, and crystallizaion in Zr41Ti14Cu12.512.5Ni10Be22.5 bulk metallic glass, Phys. Rev. B. 1998, 57: 8211.
Kissinger, H. E., Variation of peak temperature with heating rate in differential thermal analysis, J. Res. Nat. Bur. St., 1956, 57: 217.
Inoue, A., High strength bulk amorphous alloys with low critical cooing rates, Mater. Trans. JIM, 1995, 36: 866.
Busch, R., Kim, Y. J., Johnson, W. L., Thermodynamic and kinetics of the undercooled liquid and the glass transition of the Zr41.2Ti13.8Cu12.5Ni10.0Be22.5 alloy, J. Appl. Phys., 1995, 77: 4039.
Frank, W., Homer, A., Scharwaechter, P. et al., Diffusion in amorphous metallic alloys, Mater. Sci. Eng. A, 1994, 179/180: 36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuang, Y., Zhao, D., Zhang, Y. et al. Kinetics of glass transition and crystallization in multicomponent bulk amorphous alloys. Sci. China Ser. A-Math. 43, 1195–1201 (2000). https://doi.org/10.1007/BF02872198
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02872198