Abstract
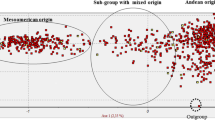
WildPhaseolus vulgaris is distributed between northern Mexico and northern Argentina. Analysis of phaseolin and molecular markers (isozymes, Restriction Fragment Length Polymorphisms or RFLPs) indicate that this gene pool consists of two major groups, Mesoamerican and Andean, and a third intermediate group found in northwestern South America. Previous to this study, only four accessions of wildP. vulgaris beans from Bolivia had been collected and their genetic relationship with other wild beans from Latin America was not known. Due to the problem of intense erosion in some areas of Bolivia, it was our objective to survey and documentPhaseolus spp. in this area before their extinction. We conducted a collection expedition in May 1994 in the departments of Cochabamba, Chuquisaca and Tarija. This resulted in collections of four populations ofP. augusti, two of cultivatedP. lunatus and two mixtures of cultivatedP. vulgaris. The first mixture was made of “k’opurus” or beans consumed after toasting, and represented an addition of 17 accessions to the Bolivian collection. The second mixture was made of “porotos” and resulted in the addition of 10 new accessions. Seven germplasm collections of wildP. vulgaris were found, which allowed us to increase the number of known populations of wild common bean for Bolivia. Another accession was found as a wild-weed-crop complex. Seven of these wildP. vulgaris accessions along with another accession from Bolivia collected previously, and a number of P. vulgaris accessions from Mexico (17), Guatemala (3), Colombia (10), Ecuador (6), Peru (17) and Argentina (16) were analyzed with RAPDs. The use of 14 random primers and one SCAR (Sequence Characterized Amplified Region) resulted in 90 bands, of which 83 were polymorphic. This data was used to construct a dendrogram which shows clear separation into three clusters, corresponding to each of the gene pools and an intermediate group. The Bolivian wild P. vulgaris beans grouped with the accessions of southern Peru and Argentina into the Andean gene pool. RAPD analysis of genetic diversity correlated well with genetic diversity obtained with other markers. Moreover, the ease of analysis allowed us to obtain a large number of bands which was conducive to greater sensitivity and identification of geographic subgroups and accessions of hybrid origin.
Resumen
Phaseolus vulgaris silvestre se encuentra distribuido entre el none de México y el none de Argentina. Mediante análisis de faseolina y marcadores moleculares (isoenzimas, RFLPs o polimorfismo de largo de fragmentos de restrictión), ha sido determinado que este acervo genético consiste de dos grupos principales, Mesoamericano y Andino, y un tercer grupo intermedio hallado en el noroeste de Sudamérica. Previo a este estudio, sólo habian sido colectadas cuatro accesiones deP. vulgaris silvestre en Bolivia, y se desconocia su relatión genética con otros frejoles silvestres de Latinoamérica. Debido al problema de erosión intensa en cienas zonas de Bolivia, nuestro objetivo fue estudiar y documentar Phaseolus spp. en esta area antes de su extinción. Realizamos una expeditión de colección en Mayo de 1994 en los depanamentos de Cochabamba, Chuquisaca y Tarija. Como resultado de ésta, coleccionamos cuatro poblaciones deP. augusti dos poblaciones de P. lunatus cultivado y dos mezclas de P. vulgaris cultivado. La primera mezcla consistió de “k’opurus,” o frejoles que son consumidos después de tostar, y representaron la adición de 17 accesiones para la colección boliviana. La segundafue una mezcla de “porotos” que resultaron en la adición de 10 nuevas accesiones. Se encontraron sie te colecciones de germoplasma de P. vulgaris silvestre, lo cual nos permitió triplicar el número de poblaciones de frejol común silvestre conocidas en Bolivia. Otra entrada fue encontrada como parte de un complejo de silvestre-maleza-cultivo. Siete de estas entradas deP. vulgaris silvestre asi como otra entrada de Bolivia colectada previamente, y varias entradas de P. vulgaris silvestre de México (17), Guatemala (3), Colombia (10), Ecuador (6), Peru (17) y Argentina (16) fueron analizadas con el uso de RAPDs (polimorfismo de DNA por amplificatión al azar). El uso de 14 cebadores y un SCAR (región amplificada de secuencia caracterizada) dieron como resultado 90 bandas, de las cuales 83 fueron polimórficas. Estos datos fueron utïlizados para la constructión de un dendrograma, que muestra clara separatión en tres grupos, que corresponden a coda uno de los acervos genéticos y un grupo intermedio. Los frejoles silvestres de Bolivia agruparon con las entradas del sur del Perú y Argentina, dentro del grupo Andino. El análisis de diversidad genética con RAPDs tuvo buena correlatión con la diversidad genética obtenida con otros marcadores. Además, la facilidad del análisis permitió obtener un gran número de bandas resultando en mayor sensitividad e identificatión de subgrupos geográficos y entradas de origen hibrido.
Similar content being viewed by others
Literature Cited
Acosta Gallegos, J. A., P. Gepts, and D. G. Debouck. 1994. Observations on wild and weedy forms of common bean in Oaxaca, Mexico. Annual Report of the Bean Improvement Cooperative 37: 137–138.
Adam-Blondon, A. F., M. Sévignac, H. Bannerot, and M. Dron. 1994. SCAR, RAPD and RFLP markers linked to a dominant gene(Are) conferring resistance to anthracnose in common bean. Theoretical and Applied Genetics 88:865–870.
Afanador, L. K., S. D. Haley, and J. D. Kelly. 1993. Adoption of a “mini-prep” DNA extraction method for RAPD marker analysis in common bean (Phaseolus vulgaris L.). Annual Report of the Bean Improvement Cooperative 36:10–11.
Baudet, J. C. 1977. The taxonomic status of the cultivated types of lima bean (Phaseolus lunatus L.). Tropical Grain Legume Bulletin, International Institute for Tropical Agriculture, Ibadan, Nigeria 7: 29–30.
Beck, S. G., T. J. Killeen, and E. Garcia E. 1993. Vegetación de Bolivia. Pages 6–24in T. J. Killeen, E. Garcia E., and S. G. Beck, eds., Guía de arboles de Bolivia. Quipus S.R.L., La Paz, Bolivia.
Becerra Velásquez, V. L., and P. Gepts. 1994. RFLP diversity in common bean (Phaseolus vulgaris L.). Genome 37:256–263.
Berglund-Brücher, O. 1967. Wildbohnen-Funde in Südamerika. Naturwissenschaft 54:466–468.
—,and H. Brücher. 1976. The south American wild bean (Phaseolus aborigineus Burk.) as ancestor of the common bean. Economic Botany 30: 257–272.
Brücher, H. 1988. The wild ancestor ofPhaseolus vulgaris in South America. Pages 185–214in P. Gepts, ed., Genetic resources ofPhaseolus beans. Kluwer Academic Publishers, Dordrecht, Holland.
Cárdenas, M. 1989. Manual de plantas económicas de Bolivia—2da edición. Editorial Los Amigos del Libro, La Paz, Bolivia.
Chase, C. D., V. M. Ortega, and C. E. Vallejos. 1991. DNA restriction fragment length polymorphisms correlate with isozyme diversity inPhaseolus vulgaris L. Theoretical and Applied Genetics 81:806–811.
Debouck, D. G. 1988a. Recolección de germoplasma dePhaseolus en Bolivia. Centro Internacional de Agricultura Tropical, Cali, Colombia, Mimeographed.
—. 1988b.Phaseolus germplasm exploration. Pages 3–29in P. Gepts, ed., Genetic resources ofPhaseolus beans. Kluwer Academic Publishers, Dordrecht, Holland.
—. 1989. Early beans (Phaseolus vulgaris L. andP. lunatus L.) domesticated for their aesthetic value? Annual Report of the Bean Improvement Cooperative 32:62–63.
—,M. Gamarra Flores, V. Ortiz Arriola, and J. Tohme. 1989. Presence of a wild-weed-crop complex inPhaseolus vulgaris L. in Peru? Annual Report of the Bean Improvement Cooperative 32: 64–65.
—,O. Toro, O. M. Paredes, W. C. Johnson, and P. Gepts. 1993. Genetic diversity and ecological distribution ofPhaseolus vulgaris (Fabaceae) in northwestern South America. Economic Botany 47:408–423.
Delgado Salinas, A., A. Bonet, and P. Gepts. 1988. The wild relative ofPhaseolus vulgaris in Middle America. Pages 163–184in P. Gepts, ed., Genetic resources ofPhaseolus beans. Kluwer Academic Publishers, Dordrecht, Holland.
dos Santos, J. B., J. Nienhuis, P. Skroch, J. Tivang, and M. K. Slocum. 1994. Comparison of RAPD and RFLP genetic markers in determining genetic similarity amongBrassica oleracea L. genotypes. Theoretical and Applied Genetics 87:909–915.
Gentry, H. S. 1969. Origin of the common bean,Phaseolus vulgaris. Economic Botany 23:55–69.
Gepts, P. 1993. The use of molecular and biochemical markers in crop evolution studies. Pages 51–94in M. K. Hecht, ed., Evolutionary biology. Volume 27. Plenum Press, New York.
—,and F. A. Bliss. 1986. Phaseolin variability among wild and cultivated common beans (Phaseolus vulgaris) from Colombia. Economic Botany 40:469–478.
—,T. C. Osborn, K. Raska, and F. A. Bliss. 1986. Phaseolin protein variability in wild forms and landraces of the common bean (Phaseolus vulgaris): evidence for multiple centers of domestication. Economic Botany 40:451–468.
Gutiérrez Salgado, A.,P. Gepts,andD. G. Debouck. 1995. Evidence for two gene pools of the lima bean,Phaseolus lunatus, in the Americas. Genetic Resources & Crop Evolution 42: in press.
Haley, S. D., P. N. Miklas, L. Afanador, and J. D. Kelly. 1994. Random amplified polymorphic DNA (RAPD) marker variability between and within gene pools of common bean. Journal of the American Society for Horticultural Science 119: 122–125.
Hallden, C., N. O. Nilsson, I. N. Rading, and T. Sall. 1994. Evaluation of RFLP and RAPD markers in a comparison ofBrassica napus breeding lines. Theoretical and Applied Genetics 88:123–128.
Halward, T., T. T. Stalker, E. LaRue, and G. Kochert. 1992. Use of single-primer DNA amplification in genetic studies of peanut (Arachis hypogaea L.). Plant Molecular Biology 18:315–325.
Heun, M., J. P. Murphy, and T. D. Phillips. 1994. A comparison of RAPD and isozyme analysis for determining the genetic relationships amongAvena sterilis L. accessions. Theoretical and Applied Genetics 87:689–696.
Howell, E. C., H. J. Newbury, R. L. Swennen, I. A. Withers, and B. V. Ford-Lloyd. 1994. The use of RAPD for identifying and classifying Musa germplasm. Genome 37:328–332.
Jaccard, P. 1908. Nouvelles recherches sur la distribution florale. Bulletin Société Vaudoise Sciences Naturelles 44:223–270.
Jain, A., S. Bhatia, S. S. Banga, S. Prakash, and M. Lakshmikumaran. 1994. Potential use of random amplified polymorphic DNA (RAPD) technique to study the genetic diversity in Indian mustard (Brassica juncea) and its relationship to heterosis. Theoretical and Applied Genetics 88:116–122.
Johnson, A. M. 1976. The climate of Peru, Bolivia and Ecuador. Pages 147–218in W. Schwerdtfeger, ed., Climates of Central and South America. Elsevier Scientific Publishing Co., Amsterdam, The Netherlands.
Kami, J. A. 1993. Molecular evolution of phaseolin. Ph.D. Dissertation. University of California, Davis.
Kami, J., V. L. Becerra Velásquez, D. G. Debouck, and P. Gepts. 1995. Identification of the presumed ancestor ofPhaseolus vulgaris. Proceedings of the National Academy of Sciences (USA) 92: 1101–1104.
Khairallah, M. M., B. B. Sears, and M. W. Adams. 1992. Mitochondrial restriction length polymorphisms in wild Phaseolus vulgaris L.: insights on the domestication of the common bean. Theoretical and Applied Genetics 84:915–922.
Koenig, R. L., and P. Gepts. 1989. Allozyme diversity inPhaseolus vulgaris: further evidence for two major centers of genetic diversity. Theoretical and Applied Genetics 78:809–817.
—,S. P. Singh, and P. Gepts. 1990. Novel phaseolin types in wild and cultivated common bean (Phaseolus vulgaris, Fabaceae). Economic Botany 44:50–60.
Koinange, E. M. K., and P. Gepts. 1992. Hybrid weakness in wildPhaseolus vulgaris L. Journal of Heredity 83:135–139.
Llaca, V. 1994. Aspects of genome evolution in thePhaseolus vulgaris complex. Ph.D. Dissertation. University of California, Davis.
Liu, Z., and G. R. Furnier. 1993. Comparison of allozyme, RFLP, and RAPD markers for revealing genetic variation within and between trembling aspen and bigtoothaspen. Theoretical and Applied Genetics 87:97–105.
Lynch, J., A. González, J. M. Tohme, and J. A. Garcia. 1992. Variation in characters related to leaf photosynthesis in wild bean populations. Crop Science 32:633–640.
Mailer, R. D., R. Scarth, and B. Fristensky. 1994. Discrimination among cultivars of rapeseed (Brassica napus L.) using DNA polymorphisms amplified from arbitrary primers. Theoretical and Applied Genetics 87:697–704.
Montes de Oca, I. 1989. Geografía y recursos naturales de Bolivia. Editorial Educational, Ministerio de Educación y Cultura, La Paz, Bolivia.
Nienhuis, J., J. Tivang, and P. Skroch. 1994. Analysis of genetic relationships among genotypes based on molecular data.In Proc. Symp. Anal. Mol. Marker Data Aug. 5-6, 1994, Corvallis, OR, Joint Plant Breed. Symp. Series, Amer. Soc. Hort. Sci., Alexandria, VA, and, Madison, WI: pp. 8–14.
Nodari, R. O., E. M. K. Koinange, J. D. Kelly, and P. Gepts. 1992. Towards an integrated linkage map of common bean. 1. Development of genomic DNA probes and levels of restriction fragment length polymorphism. Theoretical and Applied Genetics 84:186–192.
—,S. M. Tsai, R. L. Gilbertson, and P. Gepts. 1993. Towards an integrated linkage map of common bean. 2. Development of an RFLP-based linkage map. Theoretical and Applied Genetics 85: 513–520.
Orozco-Castillo, C., K. J. Chalmers, R. Waugh, and W. Powell. 1994. Detection of genetic diversity and selective gene introgression in coffee using RAPD markers. Theoretical and Applied Genetics 87:934–940.
Pearsall, D. M. 1992. The origins of plant cultivation in South America. Pages 173–205in C. W. Cowan and P. J. Watson, eds., The origins of agriculture—an international perspective. Smithsonian Institution Press, Washington, D.C.
Piper, C. V. 1926. Studies in American Phaseolinae. Contributions to the US National Herbarium 22: 663–701.
Quiros, C. F., J. Hu, P. This, A. M. Chèvre, and M. Delseny. 1991. Development and chromosomal localization of genome-specific markers by the polymerase chain reaction. Theoretical and Applied Genetics 82:627–632.
Rafalski, J. A., S. V. Tingey, and J. F. K. Williams. 1991. RAPD markers—a new technology for genetic mapping and plant breeding. Agbiotech News and Info. 3:645–648.
Ramírez E., R., D. H. Timothy, E. Diaz B., and U. J. Grant. 1960. Races of maize in Bolivia. National Academy of Sciences, National Research Council, Publication 747, Washington, D.C.
Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy. W. H. Freeman, San Francisco, California.
Thormaun, C. E., M. E. Ferreira, L. E. A. Camargo, J. G. Tivang, and T. C. Osborn. 1994. Comparison of RFLP and RAPD markers to estimating genetic relationships within and among cruciferous species. Theoretical and Applied Genetics 88:973–980.
Tohme, J., O. Toro, J. Vargas, and D. G. Debouck. 1995. Variability studies in Andeannuña common beans (Phaseolus vulgaris, Fabaceae). Economic Botany 49:78–95.
Toro, O., J. Tohme, and D. G. Debouck. 1990. Wild bean (Phaseolus vulgaris L.): description and distribution. Centre Internacional de Agriculture Tropical, Cali, Colombia.
Transite, D. K., D. J. Fairbanks, L. R. Robison, and W. R. Andersen. 1994. Species identification by RAPD analysis of grain amaranth genetic resources. Crop Science 34:1385–1389.
Unzueta, O. 1975. Mapa ecológico de Bolivia. Memoria explicativa. Ministerio de Asuntos Campesinos y Agropecuarios. División de Riegos e Ingenieria, La Paz, Bolivia.
Vierling, R. A., and H. T. Nguyen. 1992. Use of RAPD markers to determine the genetic diversity of diploid wheat genotypes. Theoretical and Applied Genetics 84:835–838.
Voysest, O. 1983. Variedades de frijol en América Latina y su origen. Centro Internacional de Agricultura Tropical, Cali, Colombia. 87 pp.
Waugh, R., and W. Powell. 1992. Using RAPD markers for crop improvement. Trends in Biotechnology 10:186–191.
Wellhausen, E. J., L. M. Roberts, and E. Hernández Xolocotzi. 1952. Races of maize in Mexico. Their origin, characteristics and distribution. Bussey Institution, Harvard University, Harvard, Massachusetts, USA.
Welsh, J., and M. McClelland. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Research 18:7213–7218.
Williams, J. G. K., A. R. Kubelik, K. J. Livak, J. A. Rafalski, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531–6535.
Yang, X., and C. Quiros. 1993. Identification and classification of celery cultivars with RAPD markers. Theoretical and Applied Genetics 86:205–212.
Yu, L. X., and H. T. Nguyen. 1994. Genetic variation detected with RAPD markers among upland and lowland rice cultivars (Oryza sativa L.). Theoretical and Applied Genetics 87:668–672.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Freyre, R., Ríos, R., Guzmán, L. et al. Ecogeographic distribution ofPhaseolus spp. (Fabaceae) in Bolivia. Econ Bot 50, 195–215 (1996). https://doi.org/10.1007/BF02861451
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02861451