Abstract
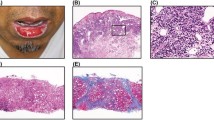
The case of a 46-year-old woman with chronic hepatitis C who was prescribed subcutaneous pegylated interferon once a week and oral ribavirin once a day is presented. Within 24 h after the first injection to her left arm, the patient developed pruritus and erythematous papules at the injection site and painful papules on her hands. After immediate administration of antihistamines, the pruritus and papules remitted. One wk later, after injection in the right arm, skin lesions and pruritus were seen. After the third injection to the abdomen, the patient developed a rash, and after the fourth and fifth injections to other areas of the abdomen, injection-site papules were seen. The patient had no skin reactions for the next 12 mo, with the exception of injection-site papules. Hepatitis C virus RNA was negative after 12 mo of treatment. Clearly, patience is important during hepatitis C therapy in order to avoid unnecessary examinations and to promote successful outcomes.
Similar content being viewed by others
References
Willems M, Munte K, Vrolijk JM, et al. Hyperpigmentation during interferon-alpha therapy for chronic hepatitis C virus infection.Br J Dermatol. 2003; 149: 390–394.
Gurguta C, Kauer C, Bergholz U, Formann E, Steindl-Munda P, Ferenci P. Tongue and skin hyperpigmentation during PEG-interferon-alpha/ribavirin therapy in dark-skinned non-Caucasian patients with chronic hepatitis C.Am J Gastroenterol. 2006; 101: 197–198.
McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C: hepatitis interventional therapy group.N Engl J Med. 1998; 339: 1485–1492.
Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.Health Technol Assess. 2004; 8:iii-iv, 1–125.
Abe S, Narita R, Oto T, Tabaru A, Otsuki M. Effect of combination therapy with ribavirin and high-dose interferon-α2b for 24 weeks in chronic hepatitis C.J Gastroenterol Hepatol. 2006; 21: 308–312.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Basaranoglu, M., Celebi, S., Karaaslan, H. et al. Case study on drug-related adverse effects of hepatitis C therapy. Adv Therapy 23, 769–771 (2006). https://doi.org/10.1007/BF02850316
Issue Date:
DOI: https://doi.org/10.1007/BF02850316