Abstract
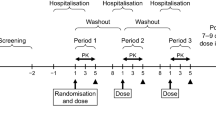
The bioavailability of β-aescin—the main active constituent of horse chestnut seed extract—in a nonretarded test medication in comparison with that in a retarded reference formulation was evaluated in 2 randomized crossover clinical trials involving 18 healthy volunteers each. Serum concentration/time curves derived under steady-state conditions and pharmacokinetic parameters measured during both studies showed no significant difference between absorption rates for the retarded versus nonretarded preparation. In the first study, investigators found a test-to-reference ratio of 1.06 (90% confidence interval [CI] range: 99–113) for the area under the curve (AUC; the primary outcome measure). Absorption rates were diminished during the night compared with daytime rates for both study preparations. In the second study, using AUC and maximum concentration (Cmax) as the primary characteristics, investigators analyzed bioavailability based on the mean of 2 consecutive daytime periods and obtained estimates of 1.07 for AUC (90% CI range: 0.96–1.19) and 1.05 for Cmax (90% CI range: 0.90–1.21). Bioequivalence of the test and reference drug preparations was thus established according to theNote for Guidance on the Investigation of Bioavaiiability and Bioequivalence. Both treatments were equally well tolerated.
Similar content being viewed by others
References
Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile.Pharmacol Res. 2001; 44:183–193.
WHO Monograph:Semen Hippocastani. Geneva: World Health Organization; 1999. WHO-TRM 07/29/98.
Dworschak E. Medical activities ofAesculum hippocastanum (horse chestnut) saponins.Adv Exp Med Biol. 1996;404:471–474.
Guillaume M, Padioleau F. Veinotonic effect, vascular protection, anti-inflammatory and free radical scavenging properties of horse chestnut extract.Arzneim Forsch/Drug Res. 1994;44:25–35.
Markward M, Loew D, eds. Venenerkrankungen. Heidelberg: Springer; 2003.
Blumenthal M, ed.The Complete German Commission E Monographs. Austin, Texas: American Botanical Council; 1998:148.
Pietta P, Mauri P, Maffei Facino R, Carini M. High-performance liquid Chromatographic analysis of β-aescin.J Chromatogr. 1989;478:259–263.
ESCOP Monograph:Hippocastani semen (Horse-chestnut seed). Exeter: European Scientific Cooperative on Phytotherapy; 1999.
Kunz K, Schaffler K, Biber A, Wauschkuhn CH. Bioverfügbarkeit von β-aescin nach oraler Gabe zweierAesculus-Extrakt enthaltender Darreichungsformen an gesunden Probanden (Bioavailability of β-aescin after oral administration of two Aesculus extract containing drug formulations to healthy volunteers).Pharmazie. 1991;46:145.
Loew D, Schrödter A, Schwankl W, März RW. Measurement of the bioavailability of aescin-containing extracts.Methods End Exp Clin Pharmacol. 2000;22:537–542.
Lehtola T, Huhtikangas A. Radioimmunoassay of aescin, a mixture of triterpene glycosides.J Immunoassay. 1990;11:17–30.
Schrader E, Schwankl W, Sieder C, Christoffel V. Vergleichende Untersuchung zur Bioverfügbarkeit von β-aescin nach oraler Einmalverabreichung zweier Roβkastanienextrakt enthaltender, galenisch unterschiedlicher Darreichungsformen.Pharmazie. 1995;50:623–627.
Oschmann R, Biber A, Lang F, Stumpf H, Kunz K. Pharmakokinetik von β-aescin nach Gabe verschiedenerAesculus-Extrakt enthaltender Formulierungen.Pharmazie. 1996;51:577–581.
Dittgen M, Zimmermann H, Wober W, Höflich C, Breitbarth H, Timpe C. Untersuchung der Bioverfügbarkeit von β-aescin nach oraler Verabreichung verschiedener Darreichungsformen.Pharmazie. 1996;51:608–610.
Kunz K, Lorkowski G, Petersen G, Samcova E, Schaffler K, Wauschkuhn CH. Bioavailability of aescin after administration of two oral formulations containingAesculus extract.Arzneim Forsch/Drug Res. 1998;48:822–825.
Schrödter A, Loew D, Schwankl W, Rietbrock N. Zur Validität radioimmunologisch bestimmter Bioverfügbarkeitsdaten von β-aescin in Roβkastaniensamenextrakten.Arzneim Forsch/Drug Res. 1998;48:905–909.
Bethge S. Zum Problem analytischer Nachweisverfahren von Aescin in Hippocastani-Semen am Beispiel einer Bioäquivalenz-Untersuchung zweier Rosskastaniensamen-Extrakt enthaltender Formulierungen.Dissertation, Johann-Wolfgang-Goethe-Universität; Frankfurt am Main, Germany; 2001.
Dingermann T, Loew D. Rosskastaniensamen Trockenextrakt: Human-Pharmakokinetik. In: Dingermann T, Loew D, eds.Phytopharmakologie. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2003:158–160.
Wagner H, Reger H, Bauer R. Saponinhaltige Drogen und Fertigarzneimittel.DAZ. 1985;25: 1513–1518.
Reger H. Die HPLC-Analyse der Saporandrogen Hippocastani semen: Primula radix und Hedera folium und daraus hergestellter Arzneipräparate.Dissertation, Ludwig-Maximilians-Universität; Munich, Germany; 1987.
Loew D, Schrödter A. Pharmakokinetik und Äquivalenz von Zubereitungen aus Hippocastani semen. In: Loew D, Blume H, Dingermann T, eds.Phytopharmaka V. Darmstadt: Steinkopff; 1999.
Loew D, Schwankl W. Validität von Immunoassays bei Wirkstoffgemischen fraglich.Pharm Ztg. 2000;145:176–183.
Committee for Proprietäry Medicinal Products (CPMP).Note for Guidance on the Investigation of Bioavailability and Bioequivalence. London; The European Agency for the Evaluation of Medicinal Products (EMEA); 2001. CPMP/EWP/QWP/1401/98.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bässler, D., Okpanyi, S., Schrödter, A. et al. Bioavailability of β-aescin from horse chestnut seed extract: Comparative clinical studies of two galenic formulations. Adv Therapy 20, 295–304 (2003). https://doi.org/10.1007/BF02849858
Issue Date:
DOI: https://doi.org/10.1007/BF02849858