Abstract
Phase relations in the ternary systems Ag2S-Cu2S-PbS and Ag2S-Cu2S-Bi2S3 were studied using the silica vacuum technique.
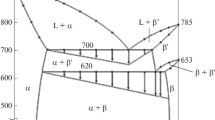
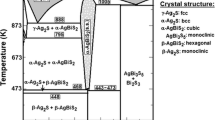
In the system Ag2S-Cu2S-Bi2S3 the phase relations are dominated by join-lines from galena to f.c.c. (Agx Cu2−xS) and b.c.c. (Cux Ag2−xS) at 500°C. With decreasing temperature, galena can coexist with all the phases on the Ag2S-Cu2S join.
There are six solid solutions, and one new phase, i.e., “C” whose composition is Ag1.1 Cu4.8Bi5.8S12 in the system Ag2S-Cu2S-Bi2S3 at 500°C. The pavonite (AgBi3S5) contains 14 mole% Cu2S in solid solution, but only 3.0 mole% Ag2S in CuBi3S5 solid solution. The Cu3Bi5S9 ss and wittichenite (Cu3BiS3) ss can form join-lines with pavonite as and have the maximum contents of 9.0 and 18 mole% Ag2S. The most striking feature is the presence of bejaminite as a stable phase with a chemical formula of Ag2Bi4S7 on the Ag2S-Bi2S3 join. AgBiS2 of the PbS type occupies a fairly large field with a maximum of 23 mole% Cu2S.
Similar content being viewed by others
References
Kullerud, G. and Yoder, H. S.,Econ. Geol., 54, 4(1959), 533–572.
Moh, G. H., and Taylor, L. A.,Neues Jahrb. Mineral., H9(1971), 405–459.
Craig, J. R. and Kullerud, G.,Amer. Mineral., 53, 1/2(1968), 145–161.
VanHook, H. J.,Econ. Geol., 55, 4(1960), 759–788.
Skinner, B. J.,Econ. Geol., 61, 1(1966), 1–26.
Ramdohr, P.,The Minerals and Their Intergrowths, Pergamon Press, Oxford, 2(1980), 481–484; 486–487.
Craig, J. R.,Mineral. Deposita, 1, 2(1967), 287–306.
Buhlmann, E.,Neues Jahrb. Mineral., 4(1971), 137–141.
Sugaki, A. and Shima, H.,Tech. Rept. Yamagushi Univ., 1(1972), 137–141.
Tomeoka, K. and Ohmasa, M.,Amer. Mineral., 67, 3/4(1982), 360–372.
Honnorez-Guerstein, B. M.,Mineral. Deposita, 6, 2(1971), 111–121.
Sugaki, A. et al.,Prof. T.Takeuchi Memorial, 1975, 73–90.
Chen, T. T. and Chang, L. L. Y.,Can. Mineral., 12, 4(1974), 404–410.
Makovicky, E. et al.,Can. Mineral., 15, 3(1977), 339–348.
Harris, D. C. and Chen, T, T.,Can. Mineral., 13, 4(1975), 400–410.
Kovalenker, V. A. and Geinke, R.V.,Bulletin of the USSR Academy of Sciences (Geology), 4(1984), 61–104(in Russian).
Karup-Moller, S.,Lithos, 9, 4(1976), 252–257.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Daqing, W. Phase relations in the systems Ag2S-Cu2S-PbS and Ag2S-Cu2S-Bi2S3 and their mineral assemblages. Chin. J. of Geochem. 6, 216–224 (1987). https://doi.org/10.1007/BF02842117
Issue Date:
DOI: https://doi.org/10.1007/BF02842117