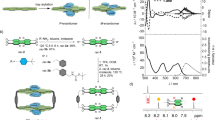
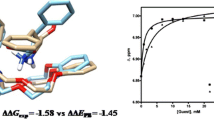
Abstract
Three new synthetic receptors based on Tröger’s base are prepared and evaluated. These three receptors are structural isomers of a successful receptor reported earlier from this laboratory, and they are all far inferior to that original receptor. Two of these receptors fail almost entirely and do so for an interesting reason. In water (not in other solvents) they collapse, forming a deflated structure. Collapsed or “sicklied” receptors do not bind to alicyclic or hydrophobic substrates in water because an important component of the driving force, the high energy waters solvating the interior of the macrocyclic structure, is lost when the receptor collapses. The results clearly illustrate how important it is to use computational methods that include good aqueous solvation models when designing new cyclophane receptors.
Similar content being viewed by others
References
Cowart M D, Sucholeiki I, Bukownik R R and Wilcox C S 1988J. Am. Chem. Soc. 110 6204
Diederich F 1988Angew. Chem. Int. Ed. Engl. 27 362
Diederich F and Dick K 1983Angew. Chem. Int. Ed. Engl. 22 715
Fischer E 1894Berichte 27 2985
Jarvi E T and Whitlock H W Jr 1980J. Am. Chem. Soc. 102 657
Mislow K and Bickart P 1976/1977Isr. J. Chem. 15 1
Mohamadi F, Richards N G J, Guida W C, Liskamp R, Caufield C, Chang G, Hendrickson T and Still W C 1990J. Comput. Chem. 11 440
Murakami Y, Sunamoto J and Kano K 1974Bull. Chem. Soc. Jpn. 47 1238
Odashima K, Itai A, Iitaka Y and Koga K 1980J. Am. Chem. Soc. 102 2504
Petti M A, Shepodd T J and Dougherty D A 1986Tetrahedron Lett. 27 807
Sucholeiki I, Lynch V, Phan L and Wilcox C S 1988J. Org. Chem. 53 98
Tabushi I, Yamada H, Matsushita K, Yoshida Z, Kuroda H and Oda R 1972Tetrahedron 28 3381
Takahashi I, Odashima K and Koga K 1985Chem. Pharm. Bull. 33 3571
Tanida H and Ishitobi H 1964Tetrahedron Lett 807
Webb T H, Suh H and Wilcox C S 1991J. Am. Chem. Soc. 113 8554
Webb T H and Wilcox C S 1990J. Org. Chem. 55 363
Wilcox C S 1985Tetrahedron Lett 26 5749
Wilcox C S 1991 InFrontiers in supramolecular organic chemistry (eds) H J Schneider and H Dürr(Weinheim, Germany: VCH) pp. 123–143
Wilcox C S and Cowart M D 1986Tetrahedron Lett. 27 5563
Wilcox C S, Webb T H, Zawacki F J, Glagovich N M and Suh H 1993Supramolecular Chem. 1 129
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glagovich, N.M., Webb, T.H., Suh, H. et al. Progress in the optimization of chiral cyclophane synthetic receptors for shape selective molecular recognition in aqueous media through hydrophobic association. Proc. Indian Acad. Sci. (Chem. Sci.) 106, 955–970 (1994). https://doi.org/10.1007/BF02841910
Issue Date:
DOI: https://doi.org/10.1007/BF02841910