Abstract
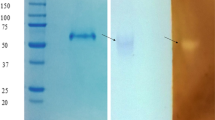
Glucoamylase produced byScytalidium thermophilum was purified 80-fold by DEAE-cellulose, ultrafiltration and CM-cellulose chromatography. The enzyme is a glycoprotein containing 9.8% saccharide, pI of 8.3 and molar mass of 75 kDa (SDS-PAGE) or 60 kDa (Sepharose 6B). Optima of pH and temperature with starch or maltose as substrates were 5.5/70 °C and 5.5/65 °C, respectively. The enzyme was stable for 1 h at 55 °C and for about 8 d at 4 °C, either at pH 7.0 or pH 5.5. Starch, amylopectin, glycogen, amylose and maltose were the substrates preferentially hydrolyzed. The activity was activated by 1 mmol/L Mg2+ (27%), Zn2+ (21%), Ba2+ (8%) and Mn2+ (5%).K m and {ie11-1} values for starch and maltose were 0.21 g/L, 62 U/mg protein and 3.9 g/L, 9.0 U/mg protein, respectively. Glucoamylase activity was only slightly inhibited by glucose up to a 1 mol/L concentration.
Similar content being viewed by others
References
Cereia M., Terenzi H.F., Jorge J.A., Greene L.J., Rosa J.C., Polizeli M.L.T.M.: Glucoamylase activity from the thermophilic fungusScytalidium thermophilum. Biochemical and regulatory properties.J. Basic Microbiol.40, 83–92 (2000).
Chakraborty K., Bhattacharyya B.K., Sen S.K.: Purification and characterization of a thermostable α-amylase fromBacillus stearothermophilus.Folia Microbiol.45, 207–210 (2000).
Crabb W.D., Mitchinson C.: Enzymes involved in the processing of starch to sugars.Trends Biotechnol.15, 349–352 (1997).
Domingues C.M., Peralta R.M.: Production of amylase by soil fungi and partial biochemical characterization of amylase of a selected strain (Aspergillus fumigatusFresenius).Can. J. Microbiol.39, 681–685 (1993).
Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F.: Colorimetric method for determination of sugars and related substances.Anal. Chem.28, 350–356 (1956).
Guzmán-Maldonado H., Paredes-López O.: Amylolytic enzymes and products derived from starch: A review.Crit. Rev. Food Sci. Nutrit.35, 373–403 (1995).
Hata Y., Ishida H., Kojima Y., Ichikawa E., Kawato A., Suginami K., Imayasu S.: Comparation of two glucoamylases produced byAspergillus oryzae in solid-state culture (Koji) and in submerged culture.J. Ferment. Bioeng.84, 532–537 (1997).
James J.A., Lee B.H.: Glucoamylases: microbial sources, industrial applications and molecular biology—a review.J. Food Biochem.21, 1–52 (1997).
Jones R.L., Verner J.E.: The bioassay of gibberelins.Plant72, 155–161 (1967).
Kiss L., Berki L.K., Nanasi P.: Evidence for a single catalytic and two binding sites in the almond emulsin β-d-glucosidase molecule.Biochem. Biophys. Res. Commun.98, 792–799 (1981).
Laemmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature227, 680–685 (1970).
Miller G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar.Anal. Chem.31, 427–429 (1959).
O'Farrell P.Z., Goodman H.M., O'Farrell P.H.: High resolution two-dimensional electrophoresis of basic as well as acidic proteins.Cell12, 1133–1142 (1977).
Pazur J.H., Liu B., Miskiel F.J.: Comparison of the properties of glucoamylases fromRhizopus niveus andAspergillus niger.Biotechnol. Appl. Biochem.12, 63–78 (1990).
Rickwood D.: An introduction to polyacrylamide gel electrophoresis, pp. 14–15 in B.D. Hames, D. Rickwood (Eds):Gel Electrophoresis of Proteins—a practical approach. IRL Press, Oxford-Washington (DC) 1985.
Silva W.B., Peralta R.M.: Purification and characterization of a thermostable glucoamylase fromAspergillus fumigatus.Can. J. Microbiol.44, 493–497 (1998).
Sivakami S., Radhakrishnan A.N.: Kinetic studies on glucoamylase of rabbit small intestine.Biochem. J.153, 321–327 (1976).
Stefanova M.E., Tonkova A.I., Dobreva E.P., Spasova D.I.: Agar gel immobilization ofBacillus brevis cells for production of thermostable α-amylase.Folia Microbiol.43, 42–46 (1998).
Straatsma G., Samson R.A.: Taxonomy ofScytalidium thermophilum, an important thermophilic fungus in mushroom compost.Mycol. Res.97, 321–328 (1993).
Thorn M.B.: A method for determining the ratio of the Michaelis constant of an enzyme with respect to two substrate.Nature4157, 27–29 (1946).
Tosi L.R.O., Terenzi H.F., Jorge J.A.: Purification and characterization of an extracellular glucoamylase from the thermophilic fungusHumicola grisea var.thermoidea.Can. J. Microbiol.39, 846–852 (1993).
Vihinen M., Mäntsälä P.: Microbial amylolytic enzymes.Crit. Rev. Biochem. Mol. Biol.24, 329–418 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aquino, A.C.M.M., Jorge, J.A., Terenzi, H.F. et al. Thermostable glucose-tolerant glucoamylase produced by the thermophilic fungusScytalidium thermophilum . Folia Microbiol 46, 11–16 (2001). https://doi.org/10.1007/BF02825876
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02825876