Abstract
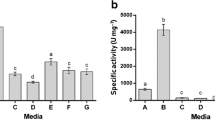
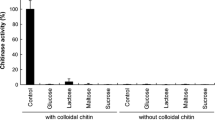
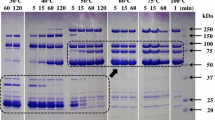
Alicyclobacillus acidocaldarius was able to degrade pectin under thermoacidophilic conditions of high temperature and acidity. Both extracellular and cell-bound pectolytic activities were found (28 and 72% of total activity, respectively). WhenA. acidocaldarius was subjected to lysozyme or sonication, more than 50% of the activity was found to be bound with the cell debris. The cell-bound enzyme presented principally exopectolytic activity. SDS-PAGE and zymogram showed that the estimated molar mass of the crude enzyme was 52 kDa. pH optimum was between 1.5 and 2.0 and the enzyme was thermostable at 70°C for 1 h at pH 2.0.
Similar content being viewed by others
References
Cruickshank R.H., Wade G.C.: Detection of pectic enzymes in pectin-acrylamide gels.Anal. Biochem.107 177–181 (1980).
Darland G., Brock C.D.:Bacillus acidocaldarius sp. nov., an acidophilic thermophilic spore-forming bacterium.J. Gen. Microbiol.67, 9–15 (1971).
Fogarty W.M., Kelly C.T.: Pectic enzymes, pp. 131–182 in W.M. Fogarty (Ed.):Microbial Enzymes and Biotechnology. Elsevier Applied Science Publishers, London 1983.
Kanno M.A.:Bacillus acidocaldarius α-amylase that is highly stable to heat under acidic conditions.Agric. Biol. Chem.50, 23–31 (1986).
Koivula T.T., Hemilä H., Pakkanen R., Sibakov M., Palva I.: Cloning and sequencing of a gene encoding acidophilic amylase fromBacillus acidocaldarius.J. Gen. Microbiol.139, 2399–2407 (1993).
Konno H., Yamasaki Y., Katoh K.: Enzymatic degradation of pectic substances and cell walls purified from carrot cell cultures.Phytochemistry25, 623–627 (1986).
Laemmli U.K.: Cleavage of structural proteins during the assembly of the head to bacteriophage T4.Nature227, 680–685 (1970).
Miller G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar.Anal. Chem.31, 56–63 (1959).
Pilnik K., Rombouts F.M.: Pectic enzymes, pp. 105–128 in J.A.V. Blancherd, J.R. Mitchel (Eds):Polysaccharidases in Food. Butterworths, London 1979.
Pilnik W.: Enzymes in the beverage industry (fruit juices, nectar, wine, spirits and beer), pp. 425–451 in CNERNA (CNRS):Use of Enzymes in Food Technology. Versailles (France) 1982.
Schwermann B., Pfau K., Liliensiek B., Schleyer M., Fischer T., Bakker E.P.: Purification, properties and structural aspects of a thermophilic α-amylase fromAlicyclobacillus acidocaldarius ATCC 27009. Insight into acidostability of proteins.Eur. J. Biochem.226, 981–991 (1994).
Wisotzkey J.D., Jurtshuk P.J.R., Fox G.E., Deinhard G., Poralla K.: Comparative sequence analysis of the 16S rRNA (rDNA) ofBacillus acidocaldarius, Bacillus acidoterrestris, andBacillus cycloheptanicus and proposal for creation of a new genus,Alicyclobacillus gen. nov.Int. J.Syst. Bacteriol.42, 263–269 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ordoñez, R.G., Morlon-Guyot, J., Gasparian, S. et al. Occurrence of a thermoacidophilic cell-bound exo-pectinase inAlicyclobacillus acidocaldarius . Folia Microbiol 43, 657–660 (1998). https://doi.org/10.1007/BF02816385
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02816385