Abstract
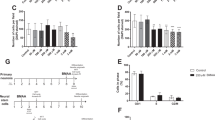
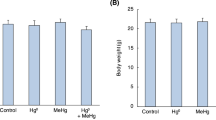
Conventionally, assessment of the neurotoxicity of environmental pollutants relies on high-dosage treatment and nonspecific end points. In the present study, the early temporal and regional alterations in the mRNAs of neurotrophins were investigated following subtoxic doses of methylmercury (MeHg) in adult Sprague-Dawley rats usingin situ hybridization histochemistry and phosphoimaging evaluation. Decreases in brain-derived neurotrophic (BDNF) mRNA labeling intensities were seen in the dentate gyrus (DG; 44% of controls), and in the CA1 (72% of controls) and CA3c (70% of controls) cell layers of hippocampus after 8 mg MeHg/kg (ip) at 4 h, and at 1 h only in the DG. The decrease in BDNF mRNA expression in the DG was dose-dependent. At 3 d, regional levels had recovered. No significant changes could be detected in mRNA levels of the BDNF high-affinity receptor trkB or neurotrophin-3 mRNA at either 1 h, 4 h, or 3 d. Cresyl violet staining and GFAP immunohistochemistry did not reveal any major neuropathology in hippocampus at 2 wk. Thus, MeHg causes specific downregulation of BDNF mRNA, unlike many other perturbations of central nervous system homeostasis that have been shown to lead to upregulation of this mRNA.
Similar content being viewed by others
References
Aschner M. and Aschner J. L. (1990) Mercury neurotoxicity: mechanisms of blood-brain barrier transport.Neurosci. Biobehav. Rev. 14, 169–176.
Aschner M., Du Y.-L., Gannon M., and Kimelberg H. K. (1993) Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures.Brain Res. 602, 181–186.
Barbany G. and Persson H. (1992) Regulation of neurotrophin mRNA in the rat brain after glucocorticoids.Eur. J. Neurosci. 4, 396–403.
Barde Y. A. (1990) The nerve growth factor family.Prog. Growth Factor Res. 2, 237–248.
Bessho Y., Nakanishi S., and Nawa H. (1993) Glutamate receptor agonists enhance the expression of BDNF mRNA in cultured cerebellar granule cells.Mol. Brain Res. 18, 201–208.
Castrén E., Pitkänen M., Sirviö J., Parsadanian A., Lindholm D., Thoenen H., and Riekkinen P. J. (1993) The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus.NeurReport 4, 895–898.
Cheung M. K. and Verity M. A. (1981) Methylmercury inhibition of synaptosomes protein synthesis: Role of mitochondrial dysfunction.Environ. Res. 24, 286–298.
Cheung M. K. and Verity M. A. (1985) Experimental methylmercury neurotoxicity: Locus of mercurial inhibition of brain protein synthesis in vivo and in vitro.J. Neurochem. 44, 1799–1808.
Choi D. W. (1988) Glutamate neurotoxicity and diseases of the nervous system.Neuron 1, 623–634.
Dagerlind Å., Friberg K., Bean A. J., and Hökfelt T. (1992) Sensitive mRNA detection using unfixed tissue: Combined radioactive and non-radioactive in situ hybridization histochemistry.Histochemistry 98, 39–49.
Ernfors P., Bengzon J., Kokai Z., Persson H., and Lindvall O. (1991) Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis.Neuron 7, 165–176.
Gall C. M. and Isackson P. J. (1989) Limbic seizures increase neuronal production of messenger RNA for nerve growth factor.Science 24, 758–761.
Hare M. F. and Atchison W. D. (1995) Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108-15 cells.J. Pharmacol. Exp. Therap. 272, 1016–1023.
Humpel C., Wetmore C., and Olson L. (1993) Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures.Neuroscience 53, 909–918.
Isackson P. J., Huntsman M. M., Murray K. D., and Gall C. M. (1991) BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF.Neuron 6, 937–948.
Komulainen H. and Tuomisto J. (1987) The neurochemical effects of methylmercury in the brain, inThe Toxicity of Methyl. Mercury (Eccles C. U. and Annau Z., eds.), The Johns Hopkins University Press, Baltimore/London, pp. 172–188.
Kung M. P., Kostyniak P. J., Olson J. R., Sansone F. M., Nickerson P. A., Malone M. A., Ziembiec N., and Roth,J. A. (1989) Cell specific enzyme markers as indicators of neurotoxicity: Effects of acute exposure to methylmercury.Neurotoxicology 10, 41–52.
Lapchak P. A., Araujo D. M., and Hefti F. (1993) Cholinergic regulation of hippocampal brain-derived neurotrophic factor mRNA expression: Evidence from lesion and chronic cholinergic drug treatment studies.Neuroscience 52, 575–585.
Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., and Barde Y. A. (1989) Molecular cloning and expression of brain-derived neurotrophic factor.Nature 341, 149–152.
Lindefors N., Ernfors P., Falkenberg T., and Persson H. (1992) Septal cholinergic afferents regulate expression of brain-derived neurotrophic factor and beta-nerve growth factor mRNA in rat hippocampus.Exp. Brain Res. 88, 78–90.
Lindvall O., Kokai Z., Bengzon J., Elmer E., and Kokai M. (1994) Neurotrophims and brain insults.Trends Neurosci. 17, 490–495.
Maissonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., and Yancopoulos G. D. (1990) Neurotrophin-3: A neurotrophic factor related to NGF and BDNF.Science 247, 1446–1451.
Middlemas D. S., Lindberg R. A., and Hunter T. (1991) TrkB, a neural receptor proteintyrosine kinase: Evidence for a full length and two truncated receptors.Mol. Cell Biol. 11, 143–153.
Nriagu J. O. (1996) A history of global metal pollution.Science 272, 223, 224.
Patterson S. L., Grover L. M., Schwartzkroin P. A., and Bothwell M. (1992) Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs.Neuron 9, 1081–1088.
Sarafian T. A., Cheung M. K., and Verity M. A. (1984) In vitro methylmercury inhibition of protein synthesis in neonatal cerebellar perikarya.Neuropathol. Appl. Neurobiol. 10, 85–100.
Thoenen H. (1995) Neurotrophins and neuronal plasticity.Science 270, 593–598.
Wetmore C. J., Olson L., and Bean A. J. (1994) Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors.J. Neurosci. 14, 1668–1700.
Zafra F., Castrén E., Thoenen H., and Lindholm, D. (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons.Proc. Natl. Acad. Sci. USA 88, 10,037–10,041.
Zafra F., Hengerer B., Leibrock J., Thoenen H., and Lindholm D. (1990) Activity-dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors.EMBO J. 9, 3545–3550.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersson, H., Lindqvist, E. & Olson, L. Downregulation of brain-derived neurotrophic factor mRNA in adult rat brain after acute administration of methylmercury. Molecular and Chemical Neuropathology 31, 225–233 (1997). https://doi.org/10.1007/BF02815126
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02815126