Abstract
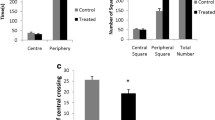
In this study, we investigated the neurobehavioral alterations and modifications of gene expression in the brains of female mice exposed to low-level mercury vapor and/or methylmercury during postnatal development. The mice were exposed to low-level mercury vapor at a mean concentration of 0.094 mg/m3 and supplied with tap water containing 5 ppm methylmercury from postnatal day 11 to 12 weeks of age. Behavioral analyses were performed at 17 weeks of age. Total locomotor activity in the open field test and the retention trial performance in the passive avoidance test were significantly reduced in the combined exposure group compared with those in the control group. The differences in locomotor activity and performance in the retention trial at 17 weeks were no longer detected at 45 weeks. These results suggest that the effect of aging on the behavioral abnormalities resulting from postnatal exposure to mercury complexes are not significant. In the microarray analysis of brains in the combined exposure group, the gene expression levels of Ano2 and Sgk1 were decreased. Real-time RT-PCR analysis confirmed these changes caused by combined mercury exposure, showing significant downregulation of Ano2 and Sgk1 in the cerebrum. These genes play key roles in the brain as a calcium-activated chloride channel and as a kinase that responds to cellular stress, respectively. Our findings provide insight into the neurobehavioral changes caused by combined mercury exposure.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
ILO (1999) Social and labor issues in small-scale in mines. Reports for the tripartite meeting on social and labor issues in small-scale in mines. International Labor Office, Geneva
Taux K, Kraus T, Kaifie A (2022) Mercury exposure and its health effects in workers in the artisanal and small-scale gold mining (ASGM) sector-a systematic review. Int J Environ Res Public Health 19:2081. https://doi.org/10.3390/ijerph19042081
Bose-O’Reilly S, Lettmeier B, Gothe RM, Beinhoff C, Siebert U, Drasch G (2008) Mercury as a serious health hazard for children in gold mining areas. Environ Res 107:89–97. https://doi.org/10.1016/j.envres.2008.01.009
Ohlander J, Huber SM, Schomaker M, Heumann C, Schierl R, Michalke B, Jenni OG, Caflisch J, Muñoz DM, von Ehrenstein OS, Radon K (2013) Risk factors for mercury exposure of children in a rural mining town in northern Chile. PLoS One 8:e79756. https://doi.org/10.1371/journal.pone.0079756
Hinton JJ, Veiga MM, Veiga ATC (2003) Clean artisanal gold mining: a utopian approach? J Clean Prod 11:99–115. https://doi.org/10.1016/S0959-6526(02)00031-8
Veiga MM, Maxson PA, Hylander LD (2006) Origin and consumption of mercury in small-scale gold mining. J Clean Prod 14:436–447. https://doi.org/10.1016/j.jclepro.2004.08.010
Counter SA, Buchanan LH (2004) Mercury exposure in children: a review. Toxicol Appl Pharmacol 198:209–230. https://doi.org/10.1016/j.taap.2003.11.032
Yoshida M, Watanabe C, Kishimoto M, Yasutake A, Satoh M, Sawada M, Akama Y (2006) Behavioral changes in metallothionein-null mice after the cessation of long-term, low-level exposure to mercury vapor. Toxicol Lett 161:210–218. https://doi.org/10.1016/j.toxlet.2005.09.007
Li P, Feng X, Qiu G, Shang L, Wang S (2008) Mercury exposure in the population from Wuchuan mercury mining area, Guizhou, China. Sci Total Environ 395:72–79. https://doi.org/10.1016/j.scitotenv.2008.02.006
Qiu G, Feng X, Li P, Wang S, Li G, Shang L, Fu X (2008) Methylmercury accumulation in rice (Oryza sativa L.) grown at abandoned mercury mines in Guizhou, China. J Agric Food Chem 56:2465–2468. https://doi.org/10.1021/jf073391a
Yoshida M, Honda A, Watanabe C, Satoh M, Yasutake A (2014) Neurobehavioral changes in response to alterations in gene expression profiles in the brains of mice exposed to low and high levels of mercury vapor during postnatal development. J Toxicol Sci 39:561–570. https://doi.org/10.2131/jts.39.561
Yoshida M, Watanabe C, Satoh M, Yasutake A, Sawada M, Ohtsuka Y, Akama Y, Tohyama C (2004) Susceptibility of metallothionein-null mice to the behavioral alterations caused by exposure to mercury vapor at human-relevant concentration. Toxicol Sci 80:69–73. https://doi.org/10.1093/toxsci/kfh138
Auer F, Franco Taveras E, Klein U, Kesenheimer C, Fleischhauer D, Möhrlen F, Frings S (2021) Anoctamin 2-chloride channels reduce simple spike activity and mediate inhibition at elevated calcium concentration in cerebellar Purkinje cells. PLoS One 16:e0247801. https://doi.org/10.1371/journal.pone.0247801
Zhang W, Schmelzeisen S, Parthier D, Frings S, Möhrlen F (2015) Anoctamin Calcium-Activated Chloride channels may modulate inhibitory transmission in the cerebellar cortex. PLoS One 10:e0142160. https://doi.org/10.1371/journal.pone.0142160
Kwon OC, Song JJ, Yang Y, Kim SH, Kim JY, Seok MJ, Hwang I, Yu JW, Karmacharya J, Maeng HJ, Kim J, Jho EH, Ko SY, Son H, Chang MY, Lee SH (2021) SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol Med 13:e13076. https://doi.org/10.15252/emmm.202013076
Lang F, Strutz-Seebohm N, Seebohm G, Lang UE (2010) Significance of SGK1 in the regulation of neuronal function. J Physiol 588:3349–3354. https://doi.org/10.1113/jphysiol.2010.190926
Fredriksson A, Dencker L, Archer T, Danielsson BR (1996) Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol Teratol 18:129–134. https://doi.org/10.1016/0892-0362(95)02059-4
Yoshida M, Suzuki M, Satoh M, Yasutake A, Watanabe C (2011) Neurobehavioral effects of combined prenatal exposure to low-level mercury vapor and methylmercury. J Toxicol Sci 36:73–80. https://doi.org/10.2131/jts.36.73
Yoshida M, Lee JY, Satoh M, Watanabe C (2018) Neurobehavioral effects of postnatal exposure to low-level mercury vapor and/or methylmercury in mice. J Toxicol Sci 43:11–17. https://doi.org/10.2131/jts.43.11
Yoshida M, Lee J-Y, Shimizu-Furusawa H, Satoh M, Watanabe C (2016) Neurobehavioral toxicity related to the exposure of weaning mice to low-level mercury vapor and methylmercury and influence of aging. Fundamental Toxicol Sci 3:185–193. https://doi.org/10.2131/fts.3.185
Yoshida M, Shimizu N, Suzuki M, Watanabe C, Satoh M, Mori K, Yasutake A (2008) Emergence of delayed methylmercury toxicity after perinatal exposure in metallothionein-null and wild-type C57BL mice. Environ Health Perspect 116:746–751. https://doi.org/10.1289/ehp.10906
Yoshida M, Watanabe C, Honda A, Satoh M, Yasutake A (2013) Emergence of delayed behavioral effects in offspring mice exposed to low levels of mercury vapor during the lactation period. J Toxicol Sci 38:1–6. https://doi.org/10.2131/jts.38.1
Funding
This research was funded by Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant in Aid for Scientific Research [C], No. 18K10029).
Author information
Authors and Affiliations
Contributions
JYL contributed to conceptualization, methodology, experiments, data analysis, and draft preparation. YM contributed to funding, conceptualization, data analysis, review, and editing. MS contributed to data analysis, review, and editing. CW contributed to data analysis, review, and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, JY., Yoshida, M., Satoh, M. et al. Neurobehavioral effects of the exposure to mercury vapor and methylmercury during postnatal period on mice. Toxicol Res. 40, 111–124 (2024). https://doi.org/10.1007/s43188-023-00210-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-023-00210-3