Abstract
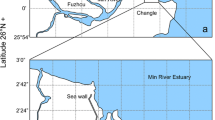
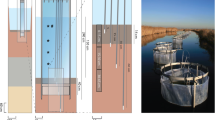
Sulfate reduction rates were measured over the course of a year in the sediments of aJuncus roemerianus marsh located in coastal Alabama. Sulfate reduction rates were typically highest in the surface 0–2 cm and at depths corresponding to peak belowground biomass of the plants. The highest volume-based sulfate reduction rate measured was 1,350 μmol liter-sediment−1 d−1 in September 1995. Areal sulfate reduction rates (integrated to 20 cm depth) were strongly correlated to sediment temperature and varied seasonally from 15.2 mmol SO 2−4 m−2 d−1 in January 1995 to 117 mmol SO 2−4 m−2 d−1 in late August 1995. Despite high sulfate reduction rates porewater dissolved sulfide concentrations were low (<73 μM), indicating rapid sulfide oxidation or precipitation. Sulfate depletion data indicated that net oxidation of sediment sulfides occurred in March through May, following a period of infrequent tidal flooding and during a period of high plant production. Porewater Fe(II) reached very high levels (maximum of 969 μM; mean for all dates was 160 μM), particularly during periods of high sulfate reduction. The annual sulfate reduction rate integrated over the upper 20 cm of sediment was 22.0 mol SO 2−4 m−2 yr−1, which is among the highest rates measured in a wetland ecosystem. Based on literature values of net primary production inJ. roemerianus marshes, we estimate that an amount equivalent to 16% to 90% of the annual belowground production may be remineralized through sulfate reduction.
Similar content being viewed by others
Literature Cited
Bradley, P. M. andJ. T. Morris. 1990 Influence of oxygen and sulfide concentrations on nitrogen uptake kinetics inSpartina alterniflora.Ecology 71:282–287.
Capone, D. G. andR. P. Kiene. 1988. Comparison of microbial dynamics in marine and freshwater sediments: Contrasts in anaerobic carbon catabolism.Limnology and Oceanography 4: 725–749.
Christian, R. R., W. L. Bryant, andM. M. Brinson. 1990.Juncus roemerianus production and decomposition along gradients of salinity and hydroperiod.Marine Ecology Progress Series 68:137–145.
Cline, J. D.. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters.Limnology and Oceanography 14: 454–458.
De la Cruz, A. A. 1974. Primary productivity of coastal marshes in Mississippi.Gulf Research Reports 4:351–356.
De la Cruz, A. A. andC. T. Hackney. 1977. Energy value, elemental composition, and productivity of belowground biomass of aJuncus tidal marsh.Ecology 58:1165–1170.
Eleuterius, L. N. 1975. The life history of the salt marsh rush,Juncus roemerianus.Bulletin Torrey Botanical Club 102:135–140.
Eleuterius, L. N.. 1976. The distribution ofJuncus roemerianus in the salt marshes of North America.Chesapeake Science 17: 289–292.
Fenchel, T., G. M. King, andT. H. Blackburn. 1998. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling, 2nd edition. Academic Press, San Diego, California.
Folk, R. L. 1974. Petrology of Sedimentary Rocks. Hemphill Publishing Company, Austin, Texas.
Fossing, H. andB. B. Jørgensen. 1989. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method.Biogeochemistry 8:205–222.
Foster, W. A.. 1968. Studies on the distribution and growth ofJuncus roemerianus in southeastern Bruswick County, in North Carolina. M.S. Thesis, North Carolina State University, Raleigh, North Carolina.
Gabriel, B. C. andA. A. De La Cruz. 1974. Species composition, standing stock and net primary production of a salt marsh community in Mississippi.Chesapeake Science 15:72–77.
Gallagher, J. L., R. J. Reimold, R. A. Linthurst, andW. J. Pfeiffer. 1980. Aerial production, mortality, and mineral accumulation-export dynamics inSpartina alterniflora andJuncus roemerianus plant stands in a Georgia salt marsh.Ecology 61: 303–312.
Giblin, A. E.. 1988. Pyrite formation in marshes during early diagenesis.Geomicrobiology Journal 6:77–97.
Hackney, C. T. andO. P. Hackney. 1978. An improved, conceptually simple technique for estimating the productivity of marsh vascular flora.Gulf Research Reports 6:125–129.
Hines, M., D. Bazylinski, J. B. Tugel, andW. B. Lyons. 1991. Anaerobic microbial biogeochemistry in sediments from two basins in the Gulf of Maine: Evidence for iron and manganese reduction.Estuarine, Coastal and Shelf Science 32:313–324.
Hines, M. E., W. L. Knollmeyer, andJ. B. Tugel. 1989. Sulfate reduction and other sedimentary biogeochemistry in a northern New England salt marsh.Limnology and Oceanography 34: 578–590.
Hopkinson, C. S., J. G. Gosselink, andR. T. Parrondo. 1980. Production of coastal Louisiana marsh plants calculated from phenometric techniques.Ecology 61:1091–1098.
Howarth, R. W.. 1978. Pyrite: Its rapid formation in a salt marsh and its importance in ecosystem metabolism.Science 203:49–51.
Howarth, R. W. andA. Giblin. 1983. Sulfate reduction in the salt marshes at Sapelo Island, Georgia.Limnology and Oceanography 28:70–82.
Howarth, R. W. andJ. M. Teal. 1979. Sulfate reduction in a New England salt marsh.Limnology and Oceanography 24:999–1013.
Howes, B. L., J. W. H. Dacey, andG. M. King. 1984. Carbon flow through oxygen and sulfate reduction pathways in salt marsh sediments.Limnology and Oceanography 29:1037–1051.
Hsieh, Y. andC. Yang. 1997. Pyrite accumulation and sulfate depletion as affected by root distribution in aJuncus (needle rush) salt marsh.Estuaries 20:640–645.
King, G.. 1990. Effects of added manganic and ferric oxides on sulfate reduction and sulfide oxidation in intertidal sediments.FEMS Microbiology Ecology 73:131–138.
King, G. M.. 1983. Sulfate reduction in Georgia salt marsh soils: And evaluation of pyrite formation by use of 35-S and 55-Fe tracers.Limnology and Oceanography 28:987–995.
King, G. M.. 1988. Patterns of sulfate reduction and the sulfur cycle in a South Carolina salt marsh.Limnology and Oceanography 33:376–390.
King, G. M., M. J. Klug, R. G. Wiegert, andA. G. Chalmers. 1982. Relation of soil water movement and sulfide concentration toSpartina alterniflora production in a Georgia salt marsh.Science 218:61–64.
Koretsky, C. M., C. M. Moore, K. L. Lowe, C. Meile, T. J. Dichristina, andP. Van Cappellen. 2003. Seasonal oscillation of microbial iron and sulfate reduction in saltmarsh sediments (Sapelo Island, GA, USA).Biogeochemistry 64:179–203.
Kostra, J. E., B. Gribsholt, E. Petrie, D. Dalton, H. Skelton, andE. Kristensen. 2002a. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments.Limnology and Oceanography 47:230–240.
Kostra, J. E. andG. W. Luther, III. 1994. Partitioning and speciation of solid phase iron in salt marsh sediments.Geochimica et Cosmochimica Acta 58:1701–1710.
Kostra, J. E., andG. W. Luther, III. 1995. Seasonal cycling of Fe in saltmarsh sediments.Biogeochemistry 29:159–181.
Kostra, J. E., A. Roychoudhury, andP. Van Cappellen. 2002b. Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments.Biogeochemistry 60:49–76.
Kruczynski, W. L., C. B. Subramanyam, andM. Drake. 1978. Studies on the plant community of a north Florida salt marsh. Part I. Primary production.Bulletin of Marine Science 28:316–334.
Lewis, D. W.. 1984. Practical Sedimentology. Hutchenson Ross, Stroudsburg, Pennsylvania.
Lord, C. J. andT. M. Church. 1983. The geochemistry of salt marshes: Sedimentary ion diffusion, sulfate reduction, and pyritization.Geochimica et Cosmochimica Acta 47:1381–1391.
Lovley, D. R.. 1987. Organic matter mineralization with reduction of ferric iron: A review.Geomicrobiology Journal 5:375–399.
Luther, G. W. andT. M. Church. 1992. An overview of the environmental chemistry of sulphur in wetland system, p. 125–142.In R. W. Howarth, J. B. W. Stewart, and M. V. Ivanov (eds.) Sulphur Cycling on the Continents. John Wiley and Sons, New York.
Luther, III,G. W., J. E. Kostka, T. M. Church, B. Sullzburger, andW. E. Stumm. 1992. Seasonal iron cycling in the salt-marsh sedimentary environments: Importance of ligand complexes with Fe(II) and Fe(III) in the dissolution of Fe(III) minerals and pyrite, respectively.Marine Chemistry 40:81–103.
Mendelssohn, I. A., K. L. McKee, andW. H. Patrick, Jr. 1981. Oxygen deficiency inSpartina alterniflora roots: Metabolic adaptations to anoxia.Science 214:439–441.
Milner, C. andR. E. Hughes. 1968. Methods for the Measurement of Primary Production of Grassland, 1st edition. Black-well Scientific Publishers, Oxford, U.K.
Mitsch, W. andJ. Gosselink. 1993. Wetlands, 2nd edition. Van Nostrand Reinhold, New York.
Moeslund, L., B. Thamdrup, andB. B. Jørgensen. 1994 Sulfur and iron cycling in a coastal sediment: Radiotracer studies and seasonal dynamics.Biogcochemistry 27:129–152.
Pomeroy, L. R. andR. G. Wiegert (eds.). 1981. The Ecology of a Salt Marsh, Ecological Studies, Volume 38. Springer-Verlag, New York.
Schroeder, W. W. andW. J. Wiseman. 1986. Low-frequency shelf-estuarine exchange processes in Mobile Bay and other estuarine systems on the Northern Gulf of Mexico, p. 355–367.In D. A. Wolfe (eds.), Estuarine Variability. Academic Press, Inc., Orlando, Florida.
Sorensen, J.. 1982. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate.Applied and Environmental Microbiology 43:319–324.
Srookey, L. L.. 1970. Ferrozine—A new spectrophotometric reagent for iron.Analytical Chemistry 42:779–781.
Stout, J. P.. 1978. An analysis of growth and productivity ofJuncus roemerianus Scheele andSpartina alterniflora Loisel in coastal Alabama. Ph.D. Dissertation, University of Alabama Tuscaloosa, Alabama.
Stroud, L. M.. 1976. Net primary production of belowground material and carbohydrate patterns in two height forms ofSpartina alterniflora in two North Carolina marshes. Ph.D. Dissertation. North Carolina State University, Raleigh, North Carolina.
Teal, J. andJ. Kanwisher. 1961. Gas exchange in a Georgia salt marsh.Limnology and Oceanography 6:388–399.
Valiela, I., J. M. Teal, andN. Y. Persson. 1976. Production and dynamics of experimentally enriched salt marsh vegetation: Belowground biomass.Limnology and Oceanography 21:245–252.
Waits, E. D.. 1967. Net primary productivity of an irregularly flooded North Carolina salt marsh. Ph.D. Dissertation. North Carolina State University, Raleigh, North Carolina.
Wetzel, R. G. 1983. Limnology, 2nd edition, Saunders, Philadelphia, Pennsylvania.
Wiegert, R. G. andF. C. Evans. 1964. Primary production and the disappearance of dead vegetation of an old field in southeastern Michigan.Ecology 45:49–62.
Willams, R. B. andM. B. Murdoch. 1972. Compartmental analysis of the production ofJuncus roemerianus in a North Carolina salt marsh.Chesapeake Science 13:69–79.
Source of Unpublished Materials
Roden, E. Personal Communication. University of Alabama, Department of Biological Sciences, Tuscaloosa, Alabama 35487.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miley, G.A., Kiene, R.P. Sulfate reduction and porewater chemistry in a gulf coastJuncus roemerianus (Needlerush) marsh. Estuaries 27, 472–481 (2004). https://doi.org/10.1007/BF02803539
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02803539