Summary
1. The paper presents a description of the new modifications of the method for eliminating the influence of interfering elements — iron, phosphorus and fluorine, — in the determination of silicic acid by the method ofDiénert andWandenbulcke.
2. The presence of large amounts of mineral acids or of their salts liable to a hydrolytic splitting, interferes with the determination, and in such cases it is recommended to add sodium acetate and acetic acid to the solution to be tested.
3. It is proposed to eliminate the influence of phosphorus and iron by adding to the coloured solution an excess of phosphoric acid, which, destroying the coloration of the phosphomolybdic complex, does not affect the intensity of the silicomolybdate and, in addition, combines with the iron to form a colourless non-dissociated phosphate.
4. The coloration of phosphomolybdate may be also eliminated by an addition of tartaric or citric acid.
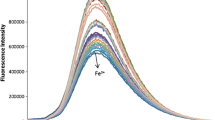
5. The influence of the fluorine ion is eliminated by in introducing aluminium chloride into the solution, which makes possible the determination of SiO2 in fluorine-bearing substances.
6. These mdoifications are specially valuable for a detection and determination of small amounts of silicic acid in fluorine salts and products of the electrolytic production of light metals and commercial phosphoric acid.
Similar content being viewed by others
Literature cited
Illingworth, J. W. andKeggin, J. F., J. Chem. Soc., London, May, 575 (1935).
Thayer, L. A., Ind. Eng. Chem., An. Ed.,2, 276 (1930).
Mêmec, A., Lanik, J. andKoppová, A. Z., Anal. chem.,83, 428 (1931).
Schwarz, M. C., Ind. Eng. Chem., An. Ed.,6, 364 (1934).
Diénert, F. andWandenbulcke, F., Compt. rend.,176, 1478 (1923); J. Chem. Soc.,124, 11, 507 (1923).
Atkins, W., J. Marine Biol. Assoc. United Kingdom.,13, 154 (1923); ibid.14, 89 (1926).
Swank, H. W. andMellon, M. G., Ind. Eng. Chem., An. Ed.,6, 348 (1934).
Pavelka, F. andMorth, H., Mikrochemie,16, 239 (1935).
King, E. J. andLucas, C. C., J. Amer. Chem. Soc.,50, 2395 (1928).
King, E. J., Ind. Eng. Chem., An. Ed.,3, 117 (1931).
Liebknecht, O., Gerb, L. andBauer, E., Z. angew. Chem.,44, 860 (1931).
Atkins, W. andWilson, E., Biochemical J.,20, 1223 (1926); C. A.,21, 1778 (1927).
Krumholz, P., Z. anorg. allg. Chem.,212, 97 (1933).
Isaacs, M. L., Bull. Soc. chim. biol.,6, 157 (1924).
Bertrand, Bull. Soc. chim. biol.,6, 656 (1924).
Feigl, F., Z. anal. Chem.,74, 389 (1928);77, 299 (1929).
Vasiliev, K. A. andBarinova, O. D., Colorimetric Determination of Silica in Metallic Aluminium and its Alloys. „Zavodskaya Laboratoria“, No. 10, 1163 (1935), (USSR.).
Author information
Authors and Affiliations
Additional information
Translated from Russian byA. S. Brashnina.
Rights and permissions
About this article
Cite this article
Alimarin, I.P., Zverev, V.S. A Modification of colorimetric determination of silicic acid in the presence of iron, phosphorus and fluorine. Mikrochemie 22, 89–101 (1937). https://doi.org/10.1007/BF02740395
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02740395