Abstract
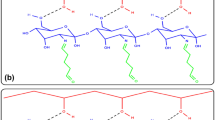
The hydrophilic copolymer, polyethylene oxide (PEO) allyl ester-maleic anhydride (MA), copolymer was used to modify the lipase from Candida rugosa. MA, in a functional group, reacts easily with amino acids of lipase. The degree of modification (DM) was varied by changing the weight ratio of copolymer to protein of lipase over the range of 10–120 (w/w). The specific activity decreased as DM increased. At the maximum modification degree of 35%, the modified lipase retained more than 65% of the unmodified native lipase activity. The modified lipase displayed a high stability of activity against temperature and pH. The remaining activity of modified lipase was about 2–4 fold of that of native lipase in the severe pH and temperature condition. Finally, it showed 20% greater reaction of substrate at 10 hr than in the case where native lipase was used.
Similar content being viewed by others
References
Benjamin, S. and Pandey, A., “Candida rugosa Lipases: Molecular Biology and Versatility in Biotechnology,”Yeast,14, 069 (1998).
Borgstrom, B. and Brockman, H. L.,Lipases, Elsevier, New York (1984).
Bousquet, M.-P., Willemot, R.-M., Monsan, P. and Boures, E., “Lipasecatalyzed α-Butylglucoside Lactate Synthesis in Organic Solvent for Dermo-cosmetic Application,”J. Biotechnol.,68, 61 (1999).
Chae, H. J., In, M. J. and Kim, E.Y., “Optimization of Protease Immobilization by Covalent Binding Using Glutaraldehyde,”Appl. Biochem. Biotech.,69, 53 (1998).
Charusheela, A. and Arvind, L., “Enzyme Catalyzed Hydrolysis of Esters Using Reversibly Soluble Polymer Conjugated Lipases,”Enzy. Mirob. Technol.,30, 19 (2002).
Diamond, A. D. and Hsu, J. T., “Aqueous Two-phase Systems for Biomolecule Separation,”Advances in Biochem. Eng. Biotech.,49, 89 (1992).
Dordick, J. S.,Biocatalysis for Industry, Plenum Press, New York (1991).
Dossat, V., Combes, D. and Marty, A., “Efficient Lipase Catalyzed Production of a Lubricant and Surfactant Formulation Using a Continuous Solvent-free Process,”J. Biotechnol.,97, 117 (2002).
Eijsink, V.G.H., BjØrk, A., Gåseidnes, S., Sirevåg, R., Synstad, B., Burg, B. denand Vriend, G., “Rational Engineering of Enzyme Stability,”J. Biotechnol.,113, 105 (2004).
Furukawa, M., Kodera, Y., Uemura, T., Hiroto, M., Matsushima, A., Kuno, H., Matsushita, H. and Inada, Y., “Alcoholysis of ε-Decalactone with Polyethylene Glycol-Modified Lipase in 1,1,1-Trichloroethane,”Biochem. and Biophys. Res. Commun.,199, 41 (1996).
Goto, M., Kamiya, N., Miyata, M. and Nakashio, F., “Enzymatic Esterification by Surfactant-coated Lipase in Organic Media,”Biotechnol. Prog.,10, 263 (1994).
Guo, Z. and Sun, Y., “Characteristics of Immobilized Lipase on Hydrophobic Superparamagnetic Microspheres to Catalyze Esterification,”Biotechnol Prog.,20, 500 (2004).
Habeeb, A. F. S. F., “Determination of Free Amino Groups in Protein by Tri-nitrobenzene Sulfonic Acid,”Anal. Biochem.,14, 328 (1966).
Jaeger, K. and Reetz, M., “Microbial Lipase Form Versatile Tools for Biotechnology,”Trends Biotechnol.,16, 396 (1998).
Jeon, G. J., Hur, B. K. and Yang, J.W., “Hydrolysis of Castor Oil with Lipases and Organic Solvents,”Korean J. Biotechnol. Bioeng.,14, 696 (1999).
Jensen, R.G., “Detection and Determination of Lipase (Acylglycerol Hydrolase) Activity from Various Sources,”Lipids,18, 650 (1983).
Joo, H., Yoo, Y. J. and Dordick, J. S., “Polymers from Biocatalysts,”Korean J. Chem. Eng.,15, 362 (1998).
Kim, B.G. and Choi, C.Y., “A Study on Ethanol Production by Immobilized Cells of Zymomonas Mobilis,”Korean J. Chem. Eng.,1, 13 (1984).
Kim, Y. S., Lee, H. J., Jang, S. K., Park, K. N., Park, J.W. and Maken, S., “Purification of Native and Modified Enzymes Using a Reactive Aqueous Two-phase System,”J. Ind. Eng. Chem.,10, 384 (2004).
Koeller, K.M. and Wong, C.-H., “Enzymes for Chemical Synthesis,”Nature,409, 232 (2001).
Kontkanen, H., Tenkanen, M., Fagerström, R. and Reinikainen, T., “Characterisation of Steryl Esterase Activities in Commercial Lipase Preparations,”J. Biotechnol.,108, 51 (2004).
Kwon, D.Y. and Rhee, J. S., “Immobilization of Lipase for Fat Splitting,”Korean J. Chem. Eng.,1, 153 (1984).
Lee, S. K., Park, S.W., Kim, Y. I., Chang, K. H., Hong, H. I. and Kim, S.W., “Immobilization of GL-7-ACA Acylase for the Production of 7-ACA,”Korean J. Chem. Eng.,19, 261 (2002).
Longo, M.A. and Combes, D., “Thermostability of Modified Enzymes: A Detailed Study,”J. Chem. Technol. Biotechnol.,74, 25 (1999).
Matsumoto, M., Kida, K. and Kondo, K., “Enhanced Activities of Lipase Pretreated with Organic Solvents,”J. Chem. Technol. Biotechnol.,76, 070 (2001).
Moon, H.Y. and Park, J.W., “Reactive Aqueous Two-phase Partition of Cellulose,”Korean J. Chem. Eng.,10, 235 (1993).
Nishio, T., Takahashi, K., Tsuzuki, T., Yoshimoto, T., Kodera, Y., Matsushima, A., Saito, Y. and Inada, Y., “Ester Synthesis in Benzene by Polyethylene Glycol-modified Lipase from Pseudomonas Fragi 22.39B,”J. Biotechnol.,8, 39 (1988).
Noel, M. and Combes, D., “Effects of Temperature and Pressure on Rhizomucor miehei Lipase Stability,”J. Biotechnol.,102, 23 (2003).
Park, C.Y., Ryu, Y.W. and Kim, C., “Kinetics and Rate of Enzymatic Hydrolysis of Cellulose in Supercritical Carbon Dioxide,”Korean J. Chem. Eng.,18, 475 (2001a).
Park, K. N. and Park, J.W., “Modification of Cellulose by Synthesized Copolymer with Polyethylene Oxide and Malic Acid Anhydride,”J. Applied Polymer Science,77, 368 (2000).
Park, J.W. and Park, K.N., “Biological De-inking of Waste Paper Using Modified Cellulose with Polyoxyethylene,”Biotechnology Techniques.,13, 49 (1999).
Park, J.W., “Chracteristics of Cellulase Modified with Amphiphilic Copolymer in Organic Solvent,”Korean J. Chem. Eng.,12, 523 (1995).
Park, J.W. and Park, K. N., “Improvement of the Physical Properties of Reprocessed Paper by Using Biological Treatment with Modified Cellulose,” Bioresource Technol.,79, 91 (2001).
Park, J.W., Park, K. N., Song, H. C. and Shin, H. C., “Saccharification and Adsoption Characteristics of Modified Cellulases with Hydrophilic/ Hydrophobic Copolymers,”J. Biotechnol.,93, 203 (2002).
Park, K. N. and Park, J.W., “Biological Reprocessing of Mixed Office Waste Using Modified Cellulase by Production of Functional Copolymer,”Korean J. Chem. Eng.,19, 285 (2002).
Park, S.W., Kim, Y. I., Chang, K. S. and Kim, S.W., “Improvement of Stability of Immobilized GL-7-ACA Acylase through Modification Glutaraldehyde,”Process Biochem.,37/2, 153 (2001b).
Reetz, M. T., “Lipases as Practical Biocatalysts,”Curr. Opin. Chem. Biol.,6, 145 (2002).
Schmid, A., Dordick, J. S., Hauer, B., Wubbolts, M. and Witholt, B., “Industrial Biocatalysis Today and Tomorrow,”Nature,409, 58 (2001).
Wu, J. C. and Song, B. D., “Esterification Reactions Catalyzed by Surfactantcoated Candida rugosa Lipase in Organic Solvents,”Process Biochem.,37, 229 (2002).
Wu, Y. X., Jaaskelainen, S. and Linko, Y.Y., “An Investigation of Crude Lipases for Hydrolysis, Esterification and Transesterification,”Enzyme Microb. Technol.,19, 226 (1996).
Wu, Z. C., He, Z.M., Yao, C.Y. and Yu, K. T., “Increased Activity and Stability of Candida rugosa Lipase in Reverse Micelles Formed by Chemically Modified AOT in Isooctane,”J. Chem. Technol. Biotechnol.,76, 949 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K., Kim, H., Maken, S. et al. Characteristics of the lipase fromCandida rugosa modified with copolymers of polyoxyethylene derivative and maleic acid anhydride. Korean J. Chem. Eng. 22, 412–417 (2005). https://doi.org/10.1007/BF02719420
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02719420