Abstract
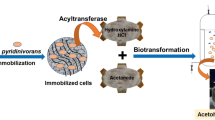
Glutaryl-7-aminocephalosporanic acid(GL-7-ACA) acylase is an important enzyme for the production of 7-ACA (7-aminocephalosporanic acid). For an efficient immobilization of GL-7-ACA acylase, various carriers were tested. A high-porous hydrophilic carrier (FPHA) among various carriers tested was found to be the best for the immobilization of GL-7-ACA acylase. In order to develop an effective immobilization method of GL-7-ACA acylase, the parameters that affect the immobilization of GL-7-ACA acylase were also investigated under different conditions of buffer solution and different concentrations of glutaraldehyde. The highest value of GL-7-ACA acylase activity (70 Unit/g-matrix) was obtained when immobilized with 1% glutaraldehyde in a 0.1 M Tris buffer (pH 8.0). Also, in order to enhance the activity of the immobilized GL-7-ACA acylase, unreacted aldehyde groups were quenched by reaction with a low molecular weight agent such as L-lysine after immobilization. The highest activity of immobilized GL-7-ACA acylase was obtained at 0.1% of L-lysine. The immobilized GL-7-ACA acylase was tested for long-term stability and it was found that the activity was retained at about 62% of the initial value after 72 times of reuse at 25 ‡C.
Similar content being viewed by others
References
Alfani, F., Cantarella, M., Cifoni, D., Spreti, N., Germani, R. and Savelli, G., “Stabilization of Acid Phosphatase in DDDACl/n-butyl Acetate Revere Micelles,”Bioprocess Eng.,21,13 (1999).
Alfani, F., Cantarella, M., Cutarella, N., Gallifuoco, A., Golini, P. and Bianchi, D., “Enzymatic Conversion of Cephalosporin C into Glutaryl 7-Aminocophalosporanic Acid. A Study in Different Reactor Configurations,”Biotech. Lett,19,175 (1997).
Bianchi, D., Golini, P., Bortolo, R., Battistel, E, Tassinari, R. and Cesti, P., “Immobilization of Glutaryl-7-ACA Acylase on Aminoalkylated Polyacrylic Supports,”Enz. Microb. Technol,20, 368 (1997).
Chae, H. J., In, M. J. and Kim, E. Y, “Optimization of Protease Immobilization by Covalent Binding Using Glutaraldehyde,”Appl. Biochem. Biotech.,73, 195 (1998).
Ezio, B., Daniele, B., Rossella, B. and Lucia, B., “Purification and Stability of Glutaryl-7-ACA Acylase fromPseudomonas sp.,”Appl. Biochem. Biotech.,69, 53 (1998).
Kim, B. G. and Choi, C. Y, “A Study on the Ethanol Production by Immobilized Cellsof Zymomonas mobilis,”Korean J. Chem. Eng.,1, 13 (1984).
Kwon, D. Y. and Rhee, J. S., “Immobilization of Lipase for Fat Splitting,”Korean J. Chem. Eng.,1, 153 (1984).
Lowry, O. H., Rosebrough, N. L., Farr, A. L. and Randall, R. J., “Protein Measurement with Folin Phenol Reagent,”J. Biol. Chem.,193, 265 (1951).
Moly Eldin, M. S., Schroen, C. G. P. H., Janssen, A. E. M., Mita, D. G. and Tramper, J., “Immobilization of Penicillin G Acylase onto Chemically Grafted Nylon Particles,”J. Mol. Catal,10,445 (2000).
Park, S. W., Kim, Y. I., Chung, K. H. and Kim, S. W., “Improvement of Stability of Immobilized GL-7-ACA Acylase through Modification with Glutaraldehyde,”Process Biochem., 37/2,153 (2001).
Roberto, F. L., Veronica, and Joes, M. G., “The Coimmobilization of D-Amino Acid Qxidase and Catalase Enables the Quantitative Transformation of D-Amino Acid (D-Phenylalanine) into α-Keto Acids (Phenylpyruvic Acid),”Enz. Microb. Technol,23,28 (1998).
Shibuya, Y, Matsumoto, K. and Fujh, T, “The Isolation and Properties ofPseudomonas Mutants with an Enhanced Productivity of 7Β-(4-Carboxybutanamido) Cephalosporanic Acid Acylase”Agric Biol. Chem.,45, 2225 (1981).
Tsuzuki, K., Komatsy, K., Ichikawa, S. and Shibuya, Y, “Enzymatic Synthesis of 7-Aminocephalosporanic Acid,”Nippon Nogeikagaku Kaishi,63,1847 (1989).
Tumuturk, H., Aksoy, S. and Hasici, N., “Covalent Immobilization of α-Amylase onto Poly(2-hydroxyethymethacrylate) and Poly(styrene-2-hydroxyethyl methacrylate) Microspheres and the Effect of Ca2+ Ions on the Enzyme Activity”Food Chem.,68, 259 (2000).
Wilhem, T. and Wedekind, F., “Immobilized Enzymes: Methods and Applications,”Top. Curr. Chem.,200,95 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SK., Park, SW., Kim, YI. et al. Immobilization of GL-7-ACA acylase for the production of 7-ACA. Korean J. Chem. Eng. 19, 261–266 (2002). https://doi.org/10.1007/BF02698411
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02698411