Abstract
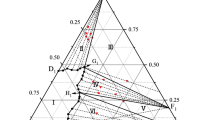
To investigate the applicability of the decorated-UNIQUAC model for multicomponent multiphase liquid mixtures, equilibrium calculations were carried out for a variety of model ternary systems, and some typical predictions were introduced. The predicted phase behavior was less limited and more complicated than that of UNIQUAC without disagreement with experimental observations on the progressional behavior of tie lines. The binodal predictions were found very sensitive with respect to the model parameters, as expected in condensed phases. The general features of nonisland type of ternary LLE were excellently reproduced by the decorated-UNIQUAC.
Similar content being viewed by others
References
Abrams, D. S. and Prausnitz, J. M.,“Statistical Thermodynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Energy of Partly or Completely Miscible Systems”,AlChEJ.,21, 116(1975).
Anderson, G. R. and Wheeler, J. C.,“Two-Component Lattice-Gas Model with a Liquid-Vapor Phase Transition in Both Pure Components”,J. Chem. Phys.,70(3), 1326 (1979).
Anderson, G. R. and Wheeler, J. C.,“Theory of Lower Critical Solution Points in Aqueous Mixtures”,J. Chem. Phys.,69(7), 3403 (1978a).
Anderson, G. R. and Wheeler, J. C.,“Directionality Dependence of Lattice Models for Solutions with Closed Loop Coexistence Curves”,J. Chem. Phys.,69(5), 2082 (1978b).
Barker, J. A.,“Cooperative Orientation Effects in Solutions”,J. Chem. Phys.,20(10), 1526 (1952).
Barker, J. A. and Fock, W.,“Theory of Upper and Lower Critical Solution Temperatures”,Disc. Faraday Soc,15, 188 (1953).
Becker, F. and Richter, P.,“Non-Aqueous Ternary Mixtures with ‘Island’ Miscibility Gaps”,Fluid Phase Equilib.,49, 157 (1989).
Burkhardt, T. W., van Leeuwen, J. M. J. (eds.),“Real-Space Renormalization”, Topics in Cuit. Phys., Vol. 30, Springer-Verlag, N.Y., 1982.
Cheluget, E. L., Weber, M. E. and Vera, J. H.,“Modifications of the Flory-Huggins-Goldstein Model for Accurate Description of Closed-Loop Phase Diagrams”,Chem. Eng. Sci.,48(8), 1415 (1993).
Elrod, M. J. and Saykally, R. J.,“Many-Body Effects in Intermolecular Forces”,Chem. Rev.,94, 1975 (1994).
Fisher, M. E.,“Transformations of Ising Models”,Physical Review,113(4), 969 (1959).
Francis, A. W., Liquid-Liquid Equilibriums”, Wiley, N.Y., 1963.
Furman, D. and Griffiths, R. B.,“Global Phase Diagram for a Van der Waals Model of a Binary Mixture”,Phys. Rev., A17, 1139(1978).
Goldstein, R. E.,“Phenomenological Theory of Multiply Reentrant Solubility”,J. Chem. Phys.,83(3), 1246 (1985).
Goldstein, R. E.,“Models for Phase Equilibria in Miscellar Solutions of Nonionic Surfactants”,J. Chem. Phys.,84(6), 3367 (1986).
Goldstein, R. E. and Walker, J. S.,“Theory of Multiple Phase Separations in Binary Mixtures: Phase Diagrams, Thermodynamic Properties, and Comparisons with Experiments”,J. Chem. Phys.,78(3), 1492 (1983).
Hand, D. B., “Dineric Distribution. I. The Distribution of a Consolute Liquid between Two Immiscible Liquids”,J. Phys. Chem.,34, 1960 (1930).
Hirschfelder, J., Stevenson, D. and Eyring, H.,“A Theory of Liquid Structure”,J. Chem. Phys.,5, 896 (1937).
Hino, T., Lambert, S. H., Soane, D. S. and Prausnitz, J. M.,“Lattice Thermodynamics for Binary Closed-Loop Equilibria: Ordinary and Polymer Systems”,AIChE J.,39(5), 837 (1993).
Hu, Y., Liu, H., Sloane, D. S. and Prausnitz, J. M., Binary Liquid-Liquid Equilibria from a Double-Lattice Model,Fluid Phase Equilib.,67, 65 (1991).
Joesten, M. and Schaad, L.,“Hydrogen Bonding”, Dekker, N. Y., 1974.
Jung, H. Y. and Jhon, M. S.,“Partial Miscibility in Water-Nicotine and Water Β-Picoline Systems”,KJChE,1, 59 (1984).
Kemeny, S., Manczinger, J., Skjold-JΦrgensen, S. and Toth, K., 447
Reduction of Thermodynamic Data by Means of the Multiresponse Maximum Likelihood Principle”,AlChE J., 28(1), 20 (1982).
Kim, Y.-C.,“liquid-Liquid Equilibria in Some Water-Pyridine Derivative Systems Using a Decorated Lattice Model”, M.S. Thesis, Dept. of Chem. Eng., KAIST (1985).
Kim, Y.-C.,“Closed-loop LLE Calculation by Lattice Decoration and Renormalization Transformation”, Ph.D. Dissertation, Dept. of Chem. Eng., KAIST (1988).
Kim, Y.-C. and Kim, J.-D.,“Decorated Lattice Model for Closed-Loop Liquid-Liquid Equilibria and its Applications to Pyridine Derivatives-Water Mixtures”,KJChE,3(2), 99 (1986).
Kim, Y.-C. and Kim, J.-D.,“New Calculation Method for Asymmetric Closed-Loop Liquid-Liquid Equilibria by Lattice Decoration”,Fluid Phase Equilib.,41, 229 (1988).
Kim, Y.-C., Kim, J.-D. and Ban, Y. B.,“Blob Calculation Method for the Liquid-Liquid Equilibria of Polymer Solutions”,Fluid Phase Equilib.,53, 331 (1989).
Kwon, Y.-S. and Park, J., A Robust Algorithm for Vapor-Liquid Equilibrium Calculations Using a Cubic Equation of State”, Personal Communication (1995).
Lang, J. C. and Morgan, R. D.,“Nonionic Surfactant Mixtures. 1. Phase Equilibria in C10E4-H2O and Closed-loop Coexistence”,J. Chem. Phys.,73(11), 5849 (1980).
Maurer, G. and Prausnitz, J. M., On the Derivation and Extension of the UNIQUAC Equation”,Fluid Phase Equilib.,2, 91 (1978).
McMahon, P. D., Glandt, E. D. and Walker, J. S.,“Renormalization Group Theory in Solution Thermodynamics,Chem. Eng. Sci.,43(10), 2561 (1988).
Meijering, J. L.,“Segregation in Regular Ternary Solutions: Part I”,Philips Res. Rep.,5, 333 (1950).
Meijering, J. L.,“Segregation in Regular Ternary Solutions: Part II”,Philips Res. Rep.,6, 183 (1951).
Mermin, N. D.. Solvable Model of Vapor-Liquid Transition with a Singular Coexistence-Curve Diameter”,Physical Review Letters,26(4), 169 (1971).
Modell, M. and Reid, R. C.,“Thermodynamics and Its Applications”, 2nd ed., Prentice-Hall, N.Y., 1983.
Mollerup, J.,“A Note on Excess Gibbs Energy Models, Equations of State and the Local Composition Concept”,Fluid Phase Equilib.,7(2), 121 (1981).
Mulholland, G. W. and Rehr, J. J.,“Coexistence Curve Properties of Mermin’s Decorated Lattice Gas”,J. Chem. Phys.,60, 1297 (1974).
Mulholland, G. W., Zollweg, J. A. and Levelt Sengers, J. M. H., “Liquid-Vapor Asymmetries in Pure Fluids”,J. Chem. Phys.,62(7), 2535 (1975).
Pimentel, G. and McClellan, A. L.,“The Hydrogen Bond”, Freeman, San Francisco, 1960.
Prausnitz, J. M., Anderson, T. F., Grens, E. A., Eckert, C. A., Hsieh, R. and O’connell, J. P.,“Computer Calculations for Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria”, Prentice-Hall, N.J., 1980.
Prausnitz, J. M., Lichtenthaler, R. N. and de Azevedo, E. G.,“Molecular Thermodynamics of Fluid-Phase Equilibria”, 2nd ed., Prentice-Hall, N.J., 1986.
Reichl, L. E., “A Modern Course in Statistical Physics”, Edward Arnold Publishers, London, 1980.
Renon, H. and Prausnitz, J. M.,“Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures”,AIChE J.,14(1), 135 (1968).
Reynolds, P. J., Klein, W. and Stanley, H. E.,“A Real-Space Renormalization Group for Site and Bond Percolation”,J. Phys. C: Solid State Phys.,10, L167 (1977).
Reynolds, P. J., Stanley, H. E. and Klein, W.,“Percolation by Position-Space Renormalization Group with Large Cells”,./.Phys. A: Math. Gen.,11(8), L199 (1978).
Reynolds, P. J., Stanley, H. E. and Klein, W.,“Large-Cell Monte Carlo Renormalization Group for Percolation”,Phys. Rev. B,21(3), 1223 (1980).
Robard, A. and Patterson, D.,“Temperature Dependence of Polystyrene-Poly(vinyl methyl ether) Compatibility in Trichloroethane”,Macromol.,10(5), 1021 (1977).
Rowlinson, J. S. and Swinton, F. L.,“Liquid and Liquid Mixtures”, 3rd ed., Butterworth Sci., London, 1959.
Sadus, R. J.,“Calculating Critical Transition of Fluid Mixtures: Theory vs. Experiment”,AIChE J.,40(8), 1376 (1994).
Sandler, S. I. (ed.),“Models for Thermodynamics and Phase Equilibria Calculations”, Univ. of Delaware, Newark, 1993.
Skjold-JΦrgensen, S., Rasmussen, P. and Fredenslund, AA, On the Temperature Dependence of the UNIQUAC/UNIFAC Models”,Chem. Eng. Sci.,35(12-B), 2389 (1980).
Treybal, R. E., Weber, L. D. and Daley, J. F.,“The System Acetone-Water-l,l,2-Trichloroethane. Ternary Liquid and Binary Vapor Equilibria”,Ind. Eng. Chem.,38, 817 (1946).
Van Konnynenberg, P. H. and Scott, R. L.,“Critical Lines and Phase Equilibria in Binary Van der Waals Mixtures,Royal Soc. of London, Phil. Trans.,A298, 1442 (1980).
Varhegyl, G. and Eon, C. H.,“Calculation of the Free Energy Equation Parameters from Ternary Liquid-Liquid Equilibrium Data”,Ind. Eng. Chem., Fundam.,16(2), 182 (1977).
Vause, C. A. and Walker, J. S.,“Effects of Orientatinal Degrees of Freedom in Closed-loop Solubility Phase Diagrams,Physics Letters,90A(8), 419 (1982).
Wegstein, J. H., Accelerating Convergence of Iterative Processes”,Commun. A.C.M.,1, 9 (1955).
Wheeler, J. C.,“Exactly Soluble Two-component Lattice Solution with Upper and Lower Critical Solution Temperatures,J. Chem. Phys.,62(2), 433 (1975).
Wheeler, J. C. and Anderson, G. R., Some Interesting New Phase Diagrams in Hydrogen-bonded Liquid Mixtures,J. Chem. Phys.,73(11), 5778 (1980).
Widom, B.,“Plait Points in Twoand Three-Component Liquid Mixtures”,J. Chem. Phys.,46(9), 3324 (1967).
Wilson, G. M.,“Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Energy of Mixing,J. Am. Chem. Soc,86, 127 (1964).
Wisniak, J.,“Liquid-Liquid Phase Splitting-II: Ternary Systems and the Spinodal Curve”,Chem. Eng. Sci,39(1), 111 (1984).
Zeman, L. and Patterson, D.,“Effect of the Solvent on Polymer Incompatibility in Solution”,Macromol,5(4), 513 (1972).
Zollweg, J. A. and Mulholland, G. W.,“On the Law of the Rectilinear Diameter”,.J. Chem. Phys.,57(3), 1021 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, YC., Kim, JD. & Kim, H. Ternary liquid-liquid phase behavior by decorated-uniquac. Korean J. Chem. Eng. 13, 439–447 (1996). https://doi.org/10.1007/BF02705991
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705991