Abstract
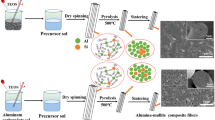
Alumina fibers were synthesized from two different systems, AlCl3-Al powder-H2O and Al(NO3)3-Al powder-H2O, by the sol-gel method. For the former system, gel fibers were obtained from solutions in a composition range of Al powder/AlCl3 molar ratios of 2 to 5. For the latter system, the spinnable range was narrower compared to the aluminum chloride system. The thermogravimetry analysis (TGA) curve of the aluminum chloride system showed a weight loss up to 700‡C while the TGA curve of the aluminum nitrate system showed no weight loss above 400‡C, which indicates that thermal decomposition of Cl- is more difficult than that of NOgk-/3.
Similar content being viewed by others
References
Bellamy, L.J., “The Infrared Spectra of Complex Molecules” Chapman Hall, London (1975).
Bitchall, J. D., “The Preparation and Properties of Polycrystalline Aluminum Oxide Fibers”Trans. J. Br. Ceram. Soc,82, 143 (1983).
Dhingra, A. K., “Alumina Fibre FP”,Phil. Trans. R. Soc, A294, 411 (1980).
Gadsden, J. A., “Infrared Spectra of Minerals and Related Inorganic Compounds” Butterworths Readings, Mass. (1975).
Gimblett, F. G. R., “Inorganic Polymer Chemistry”, Butterworths Readings, London (1963).
Hench, L. L. and Ulrich, D. R., “Ultrastructure Processing of Ceramics, Glasses and Composites” John Wiley & Sons, New York (1984).
Li, D. X. and Thomson, W. J., “Mullite Formation Kinetics of a Single-Phase Gel”J. Am. Ceram. Soc,73, 964 (1990).
Maki, T. and Sakka, S., “Preparation of Alumina Fibers by Sol-Gel Method”,J. Non-Crystal. Solids,100, 303 (1988).
Maki, T. and Sakka, S., “Preparation of Porous Alumina Fibers by Unidirectional Freezing of Gel”,J. Mater. Sci. Lett.,5, 28 (1986).
Sacks, M. D., Tseng, T. Y. and Lee, S. Y., “Thermal Decomposition of Spherical Hydrated Basic Aluminum Sulfate”Ceramic Bulletin,63, 301 (1984).
Song, K. C. and Chung, I. J., “Rheological Properties of Aluminum Hydroxide Sols during Sol-Gel Transition”,J. Non-Crystal. Solids,107, 193 (1989a).
Song, K. C. and Chung, I. J., “The Structure Formations of Aluminum Hydroxide Gels under HC1 and NH4OH Conditions”,J. Non-Crystal. Solids,108, 37 (1989b).
Song, K. C, Shim, C. S., Kang, Y. and Hong, W. G., “Preparation of Alumina Fibers from Spinnable Alumina Sols Prepared by the Sol-Gel Process”,J. of the Korean Institute of Chemical Engineers,35(1), 35 (1997).
Yogo, T. and Iwahara, H., “Synthesis of α-Alumina Fiber from Modified Aluminum Alkoxide Precursor”,J. Mater. Sci.,27, 1499 (1992).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, KC., Woo, KJ. & Kang, Y. Preparation of alumina fibers from aluminum salts by the sol-gel method. Korean J. Chem. Eng. 16, 75–81 (1999). https://doi.org/10.1007/BF02699008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02699008