Abstract
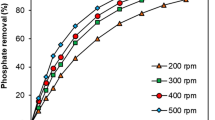
Ion exchange performance to remove nitrate in surface and underground water was studied experimentally in batch and continuous operation systems under various conditions. Data were collected by using commercially available strong-base anion-exchange resins of C1 and OH types. Equilibrium curves, obtained through the batch system and plotted as the concentration ratio versus run time, were used to evaluate the effects of temperature, resin type, and initial feed concentration on the equilibrium characteristics of nitrate. The selectivity coefficients of the resins were correlated as a function of temperature by using the Kraus-Raridon equation. Breakthrough curves, obtained through the continuous column system and plotted as the ratio of effluent to influent concentration versus solution volume passed through the experimental column, gave detailed results about the effects of the system parameters, such as temperature, resin type, feed concentration, volumetric flow rate, column diameter and height on the performance of the anion exchange to remove nitrate. The results of this study could be scaled up and used as a design tool for a water-purification system of real ground water and surface water treatment processes.
Similar content being viewed by others
References
American Water Works Association,“Standard Methods for the Examination of Water and Wastewater,” 18th Ed., Washington, D. C. (1992).
Bouchard, D. C., Williams, M. K. and Surampalli, R. Y.,“Nitrate Contamination of Groundwater: Sources and Potential Health Effects,”Journal AWWA,84(9), 85 (1993).
Clifford, D. and Liu, X.,“Ion Exchange for Nitrate Removal,”Research and Technology, Journal AWWA, 135 (1993).
Dahab, M. F.,“Comparison and Evaluation ofin-situ Bio-Denitrification Systems for Nitrate Reduction in Groundwater,”Water Science and Technology,28(3), 359 (1993).
Divekar, S. V., Foutch, G. L. and Haub, C. U.,“Mixed-Bed Ion Exchange at Concentrations Approaching the Dissociation of Water. Temperature Effects,”Industrial & Eng. Chemistry Research,26(9), 1906 (1987).
Helfferich, F. G.,“Ion Exchange”, McGraw-Hill, New York (1962).
Hill, M. J., Hawksworth, G. and Tattersall, G.,“Bacteria, Nitrosamines and Cancer of the Stomach,”British Journal of Cancer,28, 562 (1973).
Kalagiri, J. R.,“Nitrate Removal from Groundwater Using Cyclically Operated Packed-Bed Bio-Denitrification Reactors,” M.S. Thesis, University of Nebraska-Lincoln, U.S.A. (1994).
Kim, S. J., Hwang, K. R., Cho, S. Y. and Moon, H.,“Simultaneous Removal of Cyanide and Copper Ions in a Semi-Fluidized Ion Exchanger Bed,”Korean J. Chem. Eng.,16, 664 (1999).
Kraus, K. A. and Raridon, R. J.,“Temperature Dependence of Some Cation Exchange Equilibria in the Range 0 ‡C to 200 ‡C,”Journal of Physical Chemistry,63,1901 (1959).
Kraus, K. A., Raridon, R. J. and Holcomb, D. L.,“Anion Exchange Studies XXVI. A Column Method for Measurement of Ion Exchange Equilibria at High Temperature. Temperature Coefficient of the Br--Cl- Exchange Reaction,”Journal of Chromatography,3, 178 (1960).
Myers, G. E. and Boyd, G. R, “A Thermodynamic Calculation of Cation Exchange Selectivities,”Journal of Physical Chemistry,60, 521 (1956).
Pontius, F. W., “Nitrate and Cancer: Is there a Link?,”Journal AWWA, 12 (1993).
Yang, C. S., “Research on Esophageal Cancer in China: a Review,”Cancer Research,40, 2633 (1980).
Yoon, T. K., Moon, B. H. and Noh, B. I., “The Mixed-Bed Ion Exchange Performance and Temperature Effects at Ultra-Low Concentrations-1. Ion Exchange Performance,”HWAHAK KONGHAK,33, 121 (1995).
Yoon, T. K., Noh, B. I., Lee, C. W., Moon, B. H., Lee, G. C. and Jo, M. C., “The Mixed-Bed Ion Exchange Performance and Temperature Effects at Ultra-Low Concentrations-2. Temperature Effects,”Journal of Korean Industrial and Engineering Chemistry,10(2), 206 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, T., Shon, Z.H., Lee, G. et al. Parametric studies on the performance of anion exchange for nitrate removal. Korean J. Chem. Eng. 18, 170–177 (2001). https://doi.org/10.1007/BF02698455
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02698455