Abstract
Living cells create electric potential force,E, between their various phases by at least three distinct mechanisms. Charge separation,
creates the potential,\(F = \frac{Q}{{4\Pi \varepsilon _O \varepsilon _r r^2 }}\)} of -120 to -145 mV between cytoplasmic and mitochondrial phases by unbalanced proton expulsion powered by the redox energy of the respiratory chain. Electrically unbalanced flow of Na+ through voltage gated Na+ channels raises the potential of nerve from -85 to +30 mV. The so-called resting potential of cells, which varies from-85 mV in heart to -4.5 mV in red cell, does not appear to result from the unbalanced flow of ions between phases, but rather to be a measure of the work required to move ions between phases. Movement of an ion between phases entails three types of energy. Concentration work is that required to move an ion between phases containing different concentrations of ions:
Electrical work is that work required to move an ion from phases with differing electric potentials:
The Nernst potential of an ion existing at different concentrations in two phases is:
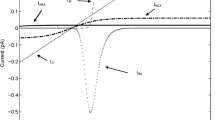
required to transport the most permeant ions in a Gibbs-Donnan near-equilibrium system, either K+ or Cl− or both, between the phases of an aqueous system during the flow of current required to measure potentials with intracellular KCl electrodes or during ion movements brought about during normal cellular activity.
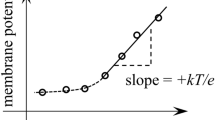
The resting electrical potential results from the existence of a mono-ionic Gibbs-Donnan near-equilibrium system between the extra- and intracellular phases of cell wherein the activity of free H2O within all phases of the system is equal and the energy of the gradients of the nine major inorganic ions, ΔG[ionz]out/in, are in near-equilibrium with one another, with the potential between the phases,E N, and with the energy of ATP hydrolysis. ΔG ATP Hydrolysis. ranges from a low of -55 to slightly over -60 kJ/mole in all cell types. While the classic inanimate Gibbs-Donnan system has only one ratio of ion concentrations between phases,r, cellular Gibbs-Donnan systems have three differentr’s representing three different equal energy ion groups or quanta, ΔG[ionz] out/in . In heart, the quanta of ΔG [Na+] out/in , [Mg2+] out/in and [H2PO −4 ] out/in was 1/3 of ΔG ATP Hydrolysis; the quanta of ΔG [H+] out/in , [HCO −3 ] out/in and [Cl−] out/in quanta was 1/3 of ΔG[Na+] out/in . The ΔG [K+] out/in in heart or ΔG[Cl−] out/in in liver is 0 and represents the resting potential.
In all cell types, a drop in the ΔG ATP Hydrolysis. resulting from anoxic or most other forms of injury decreases the extent of the Na+ gradients between extra- and intracellular phases. This leads to a steoreotypic response characterized by gain of intracellular Na+, loss of intracellular K+, decrease in resting potential and gain in intracellular H2O. A better understanding of the nature of the electrical potential between the extra- and intracellular phases of cells gives new insights into the relationship of fluid and ion changes that occur in cellular injury and apoptosis, the nature and treatment of cardiac arrhythmias and refractory seizures, as well as states of altered gene expression, such as malignant transformation and common diseases of ion transport.
Similar content being viewed by others
References
Albers R. W. (1991). Ion pumps. InEncyclopedia of Human Biology, New York: Academic Press, 557–571.
Alberts B., Bray D., Lewis J., Raff M., Roberts K., and Watson J. (1983).Molecular Biology of the Cell. New York: Garland, 1–1146.
Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., and Welsh M. J. (1991). Generation of cAMP-activated chloride currents by expression of CFRT. 251: 679–682.
Boyle P. J. and Conway E. J. (1941). Potassium accumulation in muscle and associated changes.J. Physiol. (Lond.) 100: 1–63.
Bretag A. H. (1985). Muscle chloride channels.Physiol. Rev. 67: 618–724.
Chan L., Slater J., Hasbargen J., Herndon D. N., Veech R. L., and Wolf S. (1994). Neurocardiac toxicity of racemic d,l-lactate fluids.Int. Physiol. Behav. Sci. 29: 383–394.
Chi W. M., Berezesky I. K., Smith M. W., and Trump B. F. (1995). Changes in [Ca2+]i in cultured rat proximal tubular epithelium: an in vitro model for renal ischemia.Biochim. Biophys. Acta 1243: 513–520.
Debye V. P. and Huckel E. (1923). Originalmittei, ungenzur theorie der electrolyte.Physikalische Z. 24: 185–206.
Donnan F. G. (1924). The theory of membrane equilibria.Chem. Rev. 1: 73–90.
Funder J. and Wieth J. O. (1966). Chloride and hydrogen ion distribution between human red cell and plasma.Acta Physiol. Scand 68: 234–245.
Goldman D. E. (1943). Potential, impedence, and rectification in membranes.J. Gen. Physiol. 27: 36–60.
Haldane J. B. S. (1930).Enzymes. London: Longmans, Green and Co., 74–92.
Hille B. (1992).Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 1–607.
Ho M. K. and Guidotti G. (1975). A membrane protein from human erythrocytes involved in anion exchange.J. Biol. Chem. 250: 675–683.
Hodgkin A. L. (1951). The ionic basis of electrical activity in nerve and muscle.Biol. Rev. 26: 339–409.
Huang Y. and Rane S. G. (1994). Potassium channel induction by the Ras/Raf signal transduction cascade.J Biol. Chem. 269: 31183–31189.
Kabakov A. Y. (1994). The resting potential equations incorporating ionic pumps and osmotic concentration.J. Theor. Biol. 169: 51–64.
Kanai Y., Stelzner M., Nessberger S., Khawaja S., Hebert S. C., Smith C. P., and Hediger M. A. (1994). The neuronal and epithelial human high affinity glutamate transporter.J. Biol. Chem. 269: 20599–20606.
Kashiwaya Y., Sato K., Tsuchiya N., Thomas S., Fell D. A., Veech R. L., and Passonneau J. V. (1994). Control of glucose utilization in working perfused rat heart.J. Biol. Chem. 269: 25502–25514.
Kersting U., Kersting D., and Spring K. R. (1993). Ketoconazole activates Cl- conductance and blocks Cl- and fluid adsorption by cultured cystic fibrosis (CFPAC-1) cells.Proc. Natl. Acad. Sci. U. S. A. 90: 4047–4051.
Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., and Boucher R. C. (1983). Abnormal ion permeation through cystic fibrosis respiratory epithelium. 221: 1067–1070.
Knowles M. R., Clarke L. L., and Boucher R. C. (1991). Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis.N. Engl. J. Med. 325: 533–538.
Kwack H. and Veech R. L. (1992). Citrate: its relation to free magnesium ion concentration and cellular energy. InCurrent Topics in Cellular Regulation, San Diego: Academic Press, 185–207.
Lawson J. W. R. and Veech R. L. (1979). Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions.J. Biol. Chem. 254: 6528–6537.
Leaf A. (1956). On the mechanism of fluid exchange in tissue in vitro.Biochem. J. 62: 241–248.
MacInnes D. A. (1961).Principles of Electrochemistry. New York: Dover.
Masuda T., Dobson G. P., and Veech R. L. (1990). The Gibbs-Donnan near-equilibrium system of heart.J. Biol. Chem. 265: 20321–20334.
Mitchell P. (1966). Chemiosmotic coupling in oxidative and photosynthetic phosphorylation.Biol. Rev. Camb. Philos. Soc. 41: 445–502.
Peterson G. L., Churchill L., Fisher L. E., and Hokin L. E. (1982). Structure and biosynthesis of (Na,K)-ATPase in developing brine shrimp nauplii.Ann. N. Y. Acad. Sci. 402: 185–206.
Reed B. Y. and Veech R. L. (1986). The effects of chronic administration of T4, growth hormone and epidermal growth factor on hepatic lipogenic enzymes in hypophysectomised rats.Biochem. Biophys. Res. Commun. 141: 78–83.
Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J., Drumm M. L., Iannuzzi M. C., Collins F. S., and Tsui L. (1989). Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. 245: 1066–1073.
Rojas E., Stokes C. L., Mears D., and Atwater I. (1995). Single-microelectrode voltage clamp measurements of pancreatic beta-cell membrane ionic currents in situ.J. Membr. Biol. 143: 65–77.
Roomans G. M., Von Euler A. M., and Ceder O. (1984). Microprobe analysis in studies and diagnosis of cystic fibrosis.Ann. N. Y. Acad. Sci. 428: 121–132.
Sato K., Kashiwaya Y., Keon C. A., Tsuchiya N., King M. T., Radda G. K., Chance B., Clarke K., and Veech R. L. (1995). Insulin, ketone bodies, and mitochondrial energy transduction.FASEB J. 9: 651–658.
Schwiebert E. M., Egan M. E., Hwang T., Fulmer S. B., Allen S. S., Cutting G. R., and Guggino W. B. (1995). CFTR regulates outward rectifying chloride channels through an autocrine mechanism involving ATP. 81: 1063–1073.
Stubbs M., Rodrigues L., Howe F. A., Wang J., Joeng K., Veech R. L., and Griffiths J. R. (1994). Metabolic consequences of a reversed pH gradient in rat tumors.Cancer Res. 54: 4011–4016.
Szatkowski M. and Attwell D. (1994). Triggering and execution of neuronal death in brain ischaemia: Two phases of glutamate release by different mechanisms.Trends. Neurosci. 17: 359–365.
Tabor H. and Rosenthal S. M. (1945). Effects of potassium administration, of sodium loss, and fluid loss in tourniquet shock.Public Health Rep. 60: 401–419.
Tanford C. (1950). Preparation and properties of serum plasma proteins: XXIII Hydrogen ion equilibria in native and modified human serum albumin.J. Am. Chem. Soc. 72: 441–451.
Thompson C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. 267: 1456–1462.
Van’t Hoff J. H. (1885). L’Equilibre chiminque dans les systems gazeuz ou dis a l’etal dilue.Arch. Neerl. 20: 239–302.
Veech R. L. (1986). The toxic impact of parenteral solutions on the metabolism of cells: A hypothesis for physiological parenteral therapy.Am. J. Clin. Nutr. 44: 519–551.
Veech R. L. (1988). The untoward effects of the anions of dialysis fluids.Kidney Int. 34: 587–597.
Veech R. L., Gates D. N., Crutchfield C. W., Gitomer W. L., Kashiwaya Y., King M. T., and Wondergem R. (1994). Metabolic hyperpolarization of liver by ethanol: The importance of Mg2+ and H+ in determining impermeant intracellular anionic charge and energy of metabolic reactions.Alcohol. Clin. Exp. Res. 18: 1040–1056.
Veech, R. L., King M. T., Kashiwaya Y., and Hagler H. K. 1995,Changes induced in hepatic inorganic ions distribution by ethanol or acetate. (Unpublished).
Warburg O. (1930).The Metabolism of Tumors. London: Arnold Constable.
Winslow R. L., Varghese A., Noble D., Adlakha C., and Hoythya A. (1993). Generation and propogation of extopic beats induced by spatially localized Na-K pump inhibition in atrial network models.Proc. Roy. Soc. B. 254: 55–61.
Wyllie A. H., Morris R. G., Smith A. L., and Dunlop D. (1984). Chromatin cleavage in apoptosis: Association with condensed chromatin morphology and dependence on macromolecular synthesis.J. Pathol. 142: 67–77.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Dept. of Health and Human Services, Lab. of Metabolism and Molecular Biology, National Institute on Alcohol Abuse and Alcoholism, 12501 Washington Ave., Rockville, MD 20852.
Rights and permissions
About this article
Cite this article
Veech, R.L., Kashiwaya, Y. & King, M.T. The resting membrane potential of cells are measures of electrical work, not of ionic currents. Integrative Physiological and Behavioral Science 30, 283–307 (1995). https://doi.org/10.1007/BF02691602
Issue Date:
DOI: https://doi.org/10.1007/BF02691602