Abstract
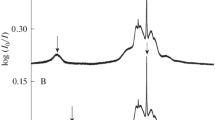
The band at 896 cm−1 in the Raman spectrum of acetic acid consists of at least two lines (873 and 896 cm−1) whose intensities and depolarization factors differ strongly. A temperature increase leads to an increase in the intensity of the 873-cm−1 line as compared to the intensity of the 896-cm−1 line. Dilution of the acid with acetonitrile leads to a similar but much more pronounced effect. In solutions with CCl4 and water, the relative intensity of the low-frequency line decreases; in aqueous solutions, the width of the 896-cm−1 line passes through a maximum with 0.4 mol. fractions of the acid (for this concentration, the line width is larger by a factor of 2.5 than in a pure liquid), while in the solutions with CCl4 it decreases smoothly (almost twofold with strong dilution: 0.05 mol. fractions). Experimental data are in agreement with the assumption that the 873- and 896-cm−1 lines refer to the vibrations of the same atoms in a molecule and to associates with a free and an H-bonded atom of the oxygen of a C=O group, respectively. The difference in the frequencies and depolarization factors of the lines causes the differences in the distribution of an electron cloud in the molecule.
Similar content being viewed by others
References
M. M. Batuev,Dokl. Akad. Nauk SSSR,53, 511–513 (1946).
N. A. Izmailov and L. M. Kuzina,Izv. Akad. Nauk SSSR Ser. Fiz.,17, 740–743 (1953).
V. M. Bilobrov,The Hydrogen Bond [in Russian], Kiev (1991).
M. M. Sushchinskii,Raman Spectra of Molecules and Crystals [in Russian], Moscow (1969).
I. V. Gerasimov and K. G. Tokhadze,Zh. Prikl. Spektrosk.,26, 1068–1072 (1977).
I. V. Gerasimov, A. I. Kul'bida, K. G. Tokhadze, and V. M. Shraiber,Zh. Prikl. Spektrosk.,32, 1066–1072 (1980).
G. V. Gusakova, G. S. Denisov, and A. L. Smolenskii,Zh. Prikl. Spektrosk.,14, 860–866 (1971).
A. L. Smolenskii,Opt. Spektrosk.,13, 475–480 (1962).
A. K. Atakhodzhaev, F. Kh. Tukhvatullin, G. Murodov, et al.,Opt. Spektrosk.,80, 208–211 (1996).
F. Kh. Tukhvatullin, A. K. Atakhodhzaev, A. A. Tursunkulov, et al.,Zh. Fiz. Khim.,6, 1032–1035 (1996).
L. M. Sverdlov, M. A. Kovner, and E. P. Krainov,Vibrational Spectra of Multiatomic Molecules [in Russian], Moscow (1970).
K. Tanabe and J. Hiraishi,Spectrochim. Acta,36A, 341–344 (1980).
Additional information
To whom correspondence should be addressed.
Alisher Navoi Samarkand State University, 15, Universitetskaya Str., Samarkand, 703004, Uzbekistan. Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 66, No. 4, pp. 467–470, July–August, 1999.
Rights and permissions
About this article
Cite this article
Tukhvatullin, F.K., Tashkenbaev, U.N., Zhumaboev, A. et al. Intermolecular hydrogen bonds in acetic acid and its solutions. J Appl Spectrosc 66, 501–505 (1999). https://doi.org/10.1007/BF02675376
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02675376