Abstract
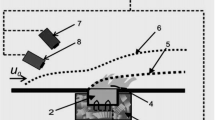
The chemical structure of a stoichiometric hydrogen-oxygen flame stabilized on a flat burner at a pressure of 47 torr is studied by molecular-beam mass spectrometry and computer simulation. Concentration profiles are measured for the stable flame components H2, O2, and H2O, as well as for the atoms and radicals H, O, and OH. The concentration profiles calculated using two different kinetic mechanisms are in acceptable agreement with experimental data. It is also shown that for sampling probes with thin walls (<-0.1 mm) at their tips, there is no need to perform the calculations using a specified temperature profile measured by thermocouples located near the aperture of the sampler, as recommended by a number of authors.
Similar content being viewed by others
References
J. Warnatz, “Calculation of the structure of laminar flat flames II: Flame velocity of freely propagating hydrogen-air and hydrogen-oxygen flames”,Ber. Bunsenges. Phys. Chem.,82, 643 (1978).
D.L. Baulch, C.J. Cobos, R.A. Cox, et al., “Evaluated kinetic data for combustion modeling”,J. Phys. Chem. Ref. Data,23, 847 (1994).
W.C. Gardiner, Jr. (ed.)Combustion Chemistry, Springer-Verlag, New York-Berlin-Heidelberg-Tokyo (1984).
N. J. Brown, K. H. Eberius, R. M. Fristrom, et al, “Low pressure hydrogen-oxygen flame studies”,Combust. Flame,33, 151 (1978).
G. Dixon-Lewis, M.M. Sutton, and A. Williams, “Flame structure and flame reaction kinetics. VII. Structure, properties, and mechanism of a rich hydrogen+nitrogen+oxygen flame at low pressure,”Proc. Roy. Soc., Ser. A,317, 227–234 (1970).
J. Vandooren and J. Bian, “Validation of H2/O2 reaction mechanisms by comparison with the experimental structure of a rich hydrogen-oxygen flame”, in:23rd Symp. (Int.) on Combustion, The Combustion Inst. Pittsburgh, PA (1990), p. 839.
J. Vandooren, V.P. Balaknin, K. Uber, and P.G. van Tiggelen, “Absolute concentration profiles of stable and labile spacies in the hydrogen-nitrous oxide front”,Kinet. Katal.,19, No. 6, 1377 (1978).
E. L. Knuth, “Composition distortion in MBMS sampling”, in:Proc. of Specialists Workshop on Applications of Free-Jet, Molecular Beam, Mass Spectrometric Sampling (October 12–14, 1994, Estes Park, CO), National Renewable Energy Laboratory (1994), pp. 18–32.
J. Vandooren and P.J. van Tiggelen, “Mass spectrometry as an efficient tool for kinetic studies in flames”,ibid., pp. 104–109.
C. Vovelle, C. Doute, and J. L. Delfau, “Mass discrimination effects in MBMS study of rich premixed flames”,ibid., pp. 187–192.
G. Dixon-Lewis, “Chemical Mechanism and properties of freely propagating hydrogen-oxygen supported flames”,Arch. Combust.,4, 279 (1984).
C.K. Westbrook, “Numerical modeling of flame inhibition by CF3Br”,Combust. Sci. Technol.,34, 201 (1983).
J.A. Miller, M.D. Smoke, and R.J. Kee, “Kinetic modeling of the oxidation of ammonia in flames”,Combust. Sci. Technol.,34, 149 (1983).
R. J. Kee, F. M. Rupley, and J. A. Miller, “CHEMKIN-II: A Fortran chemical kinetics package for the analysis of gas phase chemical kinetics”, in: Sandia Natl. Laboratories Report No. SAND89-8009B (1989).
O. P. Korobeinichev, V.M. Shvartsberg, A.A. Chernov, and V.V. Mokrushin, “Hydrogen-oxygen flame doped with trimethyl phosphate, its structure and trimethyl phosphate destruction chemistry”, in:26th Symp. (Int.) on Combustion, The Combustion Inst., Pittsburgh, PA (1996), p. 1035–1042.
O. P. Korobeinichev, S.B. Ilyin, V.V. Mokrushin, and A.G. Shmakov, “Destruction chemistry of dimethyl methylphosphonate in H2/O2/Ar flame studied by molecular beam mass spectrometry”,Combust. Sci. Technol.,116/117, 51–67 (1996).
J.P. Botha and D.B. Spalding, “The laminar flame speed of propane air mixture with heat extraction from the flame”,Proc. Roy. Soc. London, Ser. A,225, 71–96 (1954).
V.V. Dubinin, B.Ya. Kolesnikov, and G. I. Ksandopulo, “On the correctness of probe sampling in flames”,Fiz. Goreniya Vzryva,12, No. 6, 920–922 (1977).
O.P. Korobeinichev, A.G. Tereshchenko, I.D. Emel’yanov, et al., “The basis of the probe mass spectrometric method for studying the structure of flames with narrow zones”,Fiz. Goreniya Vzryva, No. 5, 22–28 (1985).
K.A. Burton, H.D. Ladouceur, and J.W. Fleming, “An improved noncatalytic coating for thermocouples”,Combust. Sci. Technol.,81, 141–145 (1992).
R. J. Kee, J. F. Grcar, M.D. Smoke, and J.A. Miller, “PREMIX”, in: Sandia Natl. Laboratories Report SAND85-8240 (1990).
NIST Standard Reference Database 25. NIST Structures and Properties Data base and Estimation Program, version 1.2, Sept. 1991; software by Stein S. E. Rukkers and R.L. Brown, Chemical Kinetics and Thermodynamics Division, NIST.
J.W. Hastie, “Sampling of reactive species from flames by mass spectrometry”,Int. J. Mass Spectron. Ion Phys.,16, 89 (1974).
Author information
Authors and Affiliations
Additional information
Translated fromFizika Goreniya i Vzryva, Vol. 35, No. 3, pp. 29–34, May–June 1999.
Rights and permissions
About this article
Cite this article
Korobeinichev, O.P., Shvartsberg, V.M., Il’in, S.B. et al. Laminar flame structure in a low-pressure premixed H2/O2/Ar mixture. Combust Explos Shock Waves 35, 239–244 (1999). https://doi.org/10.1007/BF02674444
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02674444