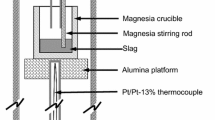
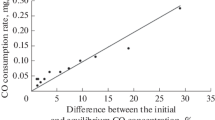
Abstract
The grain model is shown to describe satisfactorily the reduction of lead oxide and lead calcium silicate pellets by CO/CO2 gas mixtures. The reduction of lead calcium silicate, the principal lead bearing phase in the feed material to a commercial blast furnace, is initiated at about 750 °C. The activation energy is 161 kJ mol−1. These values are much higher than values for the reduction of lead oxide. The lack of chemical reaction in the upper reaches of the commercial lead blast furnace is attributed to the low reactivity of the feed material. This is a consequence of the low porosity, large particle size of the feed material, and the presence of lead in the form of complex silicates.
Similar content being viewed by others
Abbreviations
- b :
-
stoichiometric coefficient, Eq. [1]
- c 1 ,c 2 :
-
constants, Eqs. [4] and [5]c tO 1
- c o co . c o s :
-
concentration of carbon monoxide in bulk gas and of unreacted solid
- D :
-
diffusion coefficient
- D e :
-
effective diffusivity
- D m :
-
molecular diffusivity
- D k :
-
Knudsen diffusivity
- F p ,F 8 :
-
pellet and grain shape factor
- k :
-
reaction rate constant
- k p :
-
rate constant related to porosity, Eq. [13]
- M :
-
molecular mass
- N th :
-
Thiele parameter, Eq. [12]
- r :
-
grain radius
- R p :
-
pellet radius or half thickness
- R s :
-
overall rate of reaction per unit surface area, Eq. [11]
- S :
-
surface area
- S v :
-
area per unit volume
- t :
-
time
- t*:
-
dimensionless time = bkSvc ∘CO /Fgc os t
- T :
-
absolute temperature
- T m :
-
fusion temperature
- Δw :
-
weight lossX conversion
- ε :
-
porosity
- ε 0 :
-
initial porosity
- σ :
-
reaction rate modulus, Eq. [2]
- τ :
-
tortuosity
References
J.T. Chao, P.J. Dugdale, D. R. Morris, and F. R. Steward:Metall. Trans. B, 1978, vol. 9B, pp. 293–300.
D. R. Morris, B. Amero, P. G. Evans, W. Petruk, and D. R. Owens:Metall. Trans. B, 1983, vol. 14B, pp. 617–23.
J.E. Cowperthwaite, P.J. Dugdale, C.J.F Landry, D.R. Morris, F. R. Steward, and T. C. W. Wilson:Metall. Trans. B, 1980, vol. 11B, pp. 291–99.
M. M. Hussain and D.R. Morris:J. Metals, 1985, vol. 37, No. 8, pp. 57–61.
D.M. Chizhikov, Y.V. Tsvetkov, and L.G. Berezkina:Kinetika i Kataliz, 1961, vol. 2, pp. 50–54.
Y. V. Tsvetkov:Kinetika i Kataliz, 1961, vol. 2, pp. 827–29.
Y.I. Kusaev, D.M. Chizhikov, and Y. V. Tsvetkov:Tsuetnaya Metally, 1968, vol. 41, pp. 69–71.
W. A. Oates and D. D. Todd:J. Australian Inst. Metals, 1962, vol. 7, pp. 109–14.
D. K. Lambiev and M. S. Kurchatov:Kinetika i Kataliz, 1967, vol. 8, pp. 249–88.
Y. K. Rao and I.J. Lin:Can. Metall. Quart., 1975, vol. 12, pp. 125–30.
P.A. Ramachandran and L.K. Doraiswamy:A.I.Ch.E.J., 1982, vol. 28, pp. 881–900.
H.Y. Sohn and J. Szekely:Chem. Eng. Sci., 1972, vol. 27, pp. 763–79.
J. Szekely, C. I. Li, and H. Y. Sohn:Chem. Eng. Sci., 1973, vol. 28, pp. 1975–89.
J. Szekely, J. W. Evans, and H. Y. Sohn: “Gas-Solid Reactions”, Academic Press, New York, NY, 1976, pp. 115–20.
R. S. Young: “Chemical Analysis in Extractive Metallurgy”, Griffin Co. Ltd., London, 1971, pp. 181–83.
H.M. Rootare and C.F. Prenzlow:J. Phys. Chem., 1967, vol. 71, pp. 2734–36.
R.P. Mayer and R.A Stowe:J. Colloid Sci., 1965, vol. 20, pp. 894–911.
J. Szekely, J. W. Evans, and H. Y. Sohn: “Gas-Solid Reactions”, Academic Press, New York, NY, 1976, pp. 54–56.
P. A. Ramachandran and J. M. Smith:Chemical Engineering Journal, 1977, vol. 14, pp. 137–46.
L. S. Darken and R.W. Gurry: “Physical Chemistry of Metals”, McGraw-Hill Book Co., New York, NY, 1953, p. 349
A. Paulin and J.B. Mwalula:Advances in Extractive Metallurgy, 1977, Int. Symp., 3rd, pp. 97–103.
R. V. Culver, I. G. Matthew, and E. C. R. Spooner:Australian J. Chem., 1962, vol. 15, pp. 40–55.
R.B. Evans, G.M. Watson, and E. A. Mason:J. Chem.Phys., 1961, vol. 33, pp. 2076–82.
R. B. Bird, W. E. Stuart, and E. N. Lightfoot:Transport Phenomena, J. Wiley and Sons Inc., New York, NY, 1960, p. 510.
C. N. Satterfield and T. K. Sherwood:The Role of Diffusion in Catalysis, Addison Wesley, Reading, MA, 1963, p. 16.
T. C. M. Pillay and F. D. Richardson:Trans. Inst. Mining and Metall., 1956-57, vol. 66, pp. 309–29.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, M.M., Morris, D.R. Reduction of lead minerals by CO/CO2 gas mixtures: Application of the grain model. Metall Trans B 17, 575–586 (1986). https://doi.org/10.1007/BF02670225
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02670225