Abstract
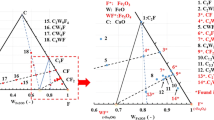
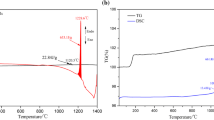
An amount of 80 mg of molten copper matte of a pseudo-ternary Cu2S-FeS-Fe system contained in a slender alumina sample tube was oxidized at 1503 and 1533 K in a mixed O2-Ar gas stream and the oxidation path was followed, comparing the overall rate of oxidation with the gaseous diffusion in the sample tube. The following successive reactions were found to be controlled by gas diffusion. Initially, Fe was oxidized to form FeO. After the melt composition reached a pseudo-ternary Cu2S-FeS-FeO system, FeS was oxidized to form FeO. As the amount of FeO increased, Fe3O4 was also formed and subsequently Cu was produced by the oxidation of Cu2S. In the latter stage, the Cu was oxidized, and the final product under the condition of gas diffusion control was composed of Cu2O, Fe3O4, and CuFeO2. On the other hand, the rate of formation of Fe2O3, CuO, and CuFe2O4 was much slower and they were not formed during the oxidation duration where the overall rate of oxidation was controlled by gas diffusion.
Similar content being viewed by others
References
S. Okada, M. Miyake, A. Hara, and M. Uekawa:Advances in Sulfide Smelting, H. Y. Sohn, D. B. George, and A. D. Zunkel, eds., TMS-AIME, Warrendale, PA, 1983, vol. 2, pp. 855–74.
T. Nagano:Physical Chemistry of Extractive Metallurgy, V. Kudryk and Y. K. Rao, eds., TMS-AIME, Warrendale, PA, 1985, pp. 311–25.
J. K. Brimacombe, A. A. Bustos, D. Jorgensen, and G. G. Richards:Physical Chemistry of Extractive Metallurgy, V. Kudryk and Y. K. Rao, eds., TMS-AIME, Warrendale, PA, 1985, pp. 327–51.
T. Rosenqvist and T. Hartvig: Royal Norwegian Council for Scientific and Industrial Research, Report 12, 1958, pp. 21–52.
M. Stofko, J. Schmiedl, and T. Rosenqvist:Scand. J. Met., 1974, vol. 3, pp. 113–18.
A. Luraschi and J.F. Elliott:Trans. Inst. Min. Met., 1980, vol. 89, pp. C14–25.
A. Yazawa, Y. Takeda, and Y. Waseda:Can. Met. Quarterly, 1981, vol. 20, pp. 129–34.
F. Ajersch and J.M. Toguri:Metall. Trans.,1972, vol. 3, pp. 2187–93.
Z. Asaki, F. Ajersch, and J. M. Toguri:Metall. Trans., 1974, vol. 5, pp. 1753–59.
R.B. Bird, W. E. Stewart, and E.N. Lightfoot:Transport Phenomena, John Wiley and Sons, Inc., New York, NY, 1960, p. 525.
R.B. Bird, W.E. Stewart, and E.N. Lightfoot:Transport Phenomena, John Wiley and Sons, Inc., New York, NY, 1960, p. 570.
N. H. Chen and D. F. Othmer:J. Chem. Eng. Data, 1962, vol. 2, pp. 37–41.
Non-ferrous Extraction Metallurgy, A. Yazawa, ed., Japan Inst. Metals, Sendai (in Japanese), 1980, pp. 315–21.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Asaki, Z., Ando, S. & Kondo, Y. Oxidation of molten copper matte. Metall Trans B 19, 47–52 (1988). https://doi.org/10.1007/BF02666489
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02666489