Abstract
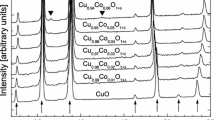
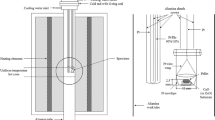
Phase relations in Cu-RO1.5-O(R < Ho,Er,Yb) ternary systems at 1273K have been established by isothermal equilibration of samples containing different ratios of Cu:R(R < Ho,Er,Yb) in flowing air or high purity argon atmosphere for four days. The samples were then rapidly cooled to ambient temperature and the coexisting phases were identified by powder x-ray diffraction analysis. Only one ternary oxide, Cu2R2O5(R < Ho,Er,Yb) was found to be stable. The chemical potential of oxygen for the coexistence of the three phase assemblage, Cu2O + R2O3 + Cu2R2O5(R < Ho,Er,Yb) has been measured by employing the solid-state galvanic cells,< (−) Pt, Cu2O + Ho2O3+ Cu2Ho2O5//CSZ//Air (Po2< 2.12 × 104 Pa), Pt (+) (−) Pt, Cu2O + Er2O3+ Cu2Er2O//CSZ//Air (Po2< 2.12 × 104 Pa), Pt (+) (−) Pt, Cu2O + Yb2O3 + Cu2Yb2O5//CSZ//Air (Po2 < 2.12 × 104 Pa), Pt (+) in the temperature range of 1000 to 1325K. Combining the measured emf of the above cells with the chemical potential of oxygen at the reference electrode, using the Nernst relationship, gives for the reactions, 2Cu2O(s) + 2Ho2O3(s) + O2(g) → 2Cu2Ho2O5(s) (1) 2Cu2O(s) + 2Er2O3(s) + O2(g) → 2Cu2Er2O5(s) (2) and 2Cu2O(s) + 2Yb2O3(s) + O2(g) → 2Cu2Yb2O5(s) (3) δΜo2 = −219,741.3 + 145.671 T (±100) Jmol−1 (4) δΜo2 = −222,959.8 + 147.98 T(±100) Jmol−1 (5) and δΜo2 = −231,225.2 + 151.847 T(±100) Jmol−1 (6) respectively. Combining the chemical potential of oxygen for the coexistence of Cu2O + R2O3 + Cu2R2O5(R− Ho,Er,Yb) obtained in this study with the oxygen potential for Cu2O + CuO equilibrium gives for the reactions, 2 CuO(s) + Ho2O3(s) → Cu2Ho2O5(s) (7) 2 CuO(s) + Er2O3(s) → Cu2Er2O5(s) (8) and 2 CuO(s) + Yb2O3(s) → Cu2Yb2O5(s) (9) δG‡ < 22,870.3 − 23.160 T (±100) Jmol−1 (10) δG‡ < 21,261.1 − 22.002 T (±100) Jmol−1 (11) and δG‡ < 17,128.4 - 20.072 T (±100) Jmol-1 (12) It can be clearly seen that the formation of Cu2R2O5R < Ho,Er,Yb) from the component oxides is endothermic. Further, Cu2R2O5(R < Ho,Er,Yb) are an entropy stabilized phases. Based on the results obtained in this study, the oxygen potential diagram for Cu-R-O(R < Ho,Er,Yb) ternary system at 1273K has been composed.
Similar content being viewed by others
References
R.J. Cava, B. Batalogg, R.B. Vandover, D.W. Murphy, S. Sunshine, T. Siegrist, J.P. Remica, E.A. Rietman, S. Zaburak and G.P. Espinosa,Phys. Rev. Lett. 58, 1676 (1987).
P.H. Hor, R.L. Meng, Y.Q. Wang, L. Gao, Z.J. Huang, J. Bechtold, K. Forster and C.W. Chu,Phys. Rev. Lett. 58, 1891 (1987).
M. Ishikawa, T. Takabatake and Y. Nakkazawa,Phys. B+C, 148 (1–3), 332 (1987).
P. Somasundaram, A.M. Raln, A.M. Umarji and C.N.R. Rao,Mater. Res. Bull. 25, 331 (1990).
T. Kogure, R. Kontra and J.B.V. Sande,Phys. C, 156, 35 (1988).
D.E. Morris, N.G. Asmar, J.H. Nickel, R.L. Sid, J.Y.T. Wei and J.E. Post,Phys. C 159, 287 (1989).
D.E. Morris, N.G. Asmar, J.Y.T. Wei, R.L. Sid, J.H. Nickel, J.S. Scott and J.E. Post,Phys. C 162–164, 955 (1989).
J. Karpinski, E. Kaldis, E. Jilek, S. Rusiecki and B. Bücher,Nature 336, 660(1988).
S. Adachi, H. Abachi, K. Setsune and K. Wasa,Phys. C, 175, 523 (1991).
G.M. Kale and K.T. Jacob,Ckem. Mater. 1, 515 (1989).
G.M. Kale and D.J. Fray, University of Leeds, Leeds, unpublished research, 1992.
G.M. Kale and D.J. Fray,Metall. Mater. Trans. B 25B, 373 (1994).
M.W. Chase, Jr., C.A. Davies, J.R. Downey, Jr., D.J. Frurip, R.A. McDonald and A.N. Syerud, JANAF Thermochemical Tables, 3rd ed., American Chemical Society and American Institute of Physics, National Bureau of Standards, Gaithersburg, MD, 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kale, G.M., Fray, D.J. Phase relations in Cu- RO1.5- O (R < Ho, Er, Yb) and gibbs energy of formation of Cu2R2O5 (R < Ho, Er, Yb) between 1000 and 1325K. J. Electron. Mater. 24, 1981–1989 (1995). https://doi.org/10.1007/BF02653021
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02653021